NEWS RELEASES
FOR IMMEDIATE RELEASE
March 17, 2005
Tiny porphyrin tubes developed by Sandia may lead to new nanodevices
Research could result in clean, inexpensive hydrogen fuel
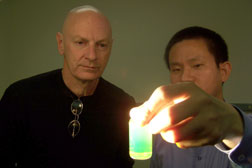 GLOWING PORPHYRIN NANOTUBES -- Sandia researchers John Shelnutt
and Zhongchun Wang gaze upon the glow of porphryin nanotubes
caused by the nanotubes’ intense resonance light scattering
activity. (Photo by Chris Burroughs)
GLOWING PORPHYRIN NANOTUBES -- Sandia researchers John Shelnutt
and Zhongchun Wang gaze upon the glow of porphryin nanotubes
caused by the nanotubes’ intense resonance light scattering
activity. (Photo by Chris Burroughs)Download 300dpi JPEG image “shelnutt.jpg,” 644K (Media are welcome to download/publish this image with related news stories.)
ALBUQUERQUE, N.M. — Sunlight splitting water molecules to produce hydrogen using devices too small to be seen in a standard microscope. That’s a goal of a research team from the National Nuclear Security Administration’s Sandia National Laboratories. The research has captured the interest of chemists around the world pursuing methods of producing hydrogen from water.
“The broad objective of the research is to design and fabricate new types of nanoscale devices,” says John Shelnutt, Sandia research team leader. “This investigation is exciting because it promises to provide fundamental scientific breakthroughs in chemical synthesis, self-assembly, electron and energy transfer processes, and photocatalysis. Controlling these processes is necessary to build nanodevices for efficient water splitting, potentially enabling a solar hydrogen-based economy.”
The prospect of using sunlight to split water at the nanoscale
grew out of Shelnutt’s research into the development
of hollow porphyrin nanotubes. (See “Porphyrin nanotubes
versus carbon nanotubes”
below.) These light-active nanotubes can be engineered to
have minute deposits of platinum and other metals and semiconductors
on the outside or inside of the tube.
 AN ELECTRON MICROSCOPE image of a porphyrin nanotube.
AN ELECTRON MICROSCOPE image of a porphyrin nanotube.Download 300dpi JPEG image, “nanotubes.jpg,” 684K (Media are welcome to download/publish this image with related news stories.)
These hollow structures are one member of a new class of nanostructures made of porphyrins that Shelnutt and his team are developing. The porphyrin building blocks (tectons) can be altered to control their structural and functional properties.
Shelnutt says these porphyrin nanotubes have “interesting electronic and optical properties such as an intense resonance light scattering ability and photocatalytic activity.” When exposed to light, some porphyrin nanotubes can photocatalytically grow metal structures onto tube surfaces to create a functional nanodevice. For example, when the nanotubes are put into a solution with gold or platinum ions and exposed to sunlight, their photocatalytic activity causes the reduction of the ions to the metal. Using this method the researchers have deposited platinum outside the nanotube and grown a nanowire of gold inside the tube.
The nanotube with the gold inside and platinum outside is the heart of a nanodevice that may split water into oxygen and hydrogen. The research team has already demonstrated that the nanotubes with platinum particles on the surface can produce hydrogen when illuminated with light. To complete the nanodevice that splits water, a nanoparticle of an inorganic photocatalyst that produces oxygen must be attached to the gold contact ball that naturally forms at the end of the tube. The gold nanowire and ball serve as a conductor of electrons between the oxygen- and hydrogen- producing components of the nanodevice. The gold conductor also keeps the oxygen and hydrogen parts separate to prevent damage during operation.
“Laboratory-scale devices of this type have already been built by others,” Shelnutt says. “What we are doing is reducing the size of the device to reap the benefits of the nanoscale architecture.”
Shelnutt says the nanodevice could efficiently use the entire visible and ultraviolet parts of the solar spectrum absorbed by the tubes to produce hydrogen, one of the Holy Grails of chemistry.
These nanotube devices could be suspended in a solution and used for photocatalytic solar hydrogen production.
“Once we have functional nanodevices that operate with reasonable efficiency in solution, we will turn our attention to the development of nanodevice-based solar light-harvesting cells and the systems integration issues involved in their production,” Shelnutt says. “There are many possible routes to the construction of functional solar cells based on the porphyrin nanodevices. For example, we may fabricate nanodevices in arrays on transparent surfaces, perhaps on a masked free-standing film. However, we have a lot of issues to resolve before we get to that point.”
Water-splitting is just one of the possible applications
of the nanodevices based on porphyrin nanostructures. Shelnutt
expects the tubes to have uses as conductors, semiconductors,
and photoconductors, and to have other properties that permit
them to be used in electronic and photonic devices and as
chemical sensors.
The work was partially funded by a grant to the University
of Georgia from the Department of Energy, Basic Energy Sciences,
Division of Chemical Sciences, Geosciences, and Biosciences.
Porphyrin nanotubes versus carbon nanotubes
Porphyrins are light-absorbing molecules related to chlorophyll, the active part of photosynthetic proteins and light-harvesting nanostructures (chlorosomal rods). They are the active molecules in many other proteins such as hemoglobin, which gets its intense red color from a porphyrin.
Porphyrin nanotubes are made entirely of oppositely charged
porphyrin molecules that self-assemble in water at room
temperature. The more well-known carbon nanotubes are formed
at high temperatures and have covalent bonds between carbon
atoms.
Porphyrin nanotubes lack the high mechanical strength of
the carbon tubes but possess a wider range of optical and
electronic properties that can be exploited in making nanodevices.
In fact, carbon nanotubes are often modified by attaching
porphyrins to increase their utility. This is unnecessary
for the porphyrin nanotubes, which can be tailored to specific
purposes like water-splitting by varying the type of porphyrin
incorporated into the nanotube itself to obtain the desired
properties.
Other porphyrin nanostructures such as nanofibers and rectangular cross-section nanotubes have been made and can also be used in the fabrication of nanodevices.
Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin company, for the U.S. Department of Energy’s National Nuclear Security Administration. Sandia has major R&D responsibilities in national security, energy and environmental technologies, and economic competitiveness.
Sandia media contact: Chris Burroughs, coburro@sandia.gov, (505) 844-0948
Sandia technical contact: John Shelnutt, jasheln@sandia.gov, (505) 272-7160