Download 300dpi JPEG image, ‘heme.jpg’, 608K
ALBUQUERQUE, N.M. — A discovery linking the shape of a unit called the heme in a protein to protein function may prove useful in a range of scientific advances, including finding cures for diseases and cleaning up pollutants, says discoverer John Shelnutt, a physicist at the Department of Energy’s Sandia National Laboratories. The first time such a correlation has been made, the discovery is being heralded by the biochemical and biophysical communities as one of the most intriguing new findings about proteins in recent years.
Proteins — strings of amino acids — are found in all living cells and are where the work of a cell occurs. The heme, which consists of a ring of carbons and nitrogens, is the portion of a “hemoprotein” that clasps the protein’s iron atom in place. A single protein may contain as few as one heme or as many as ten.
“Before, people always thought the heme’s primary function was to simply hold the iron in the protein so that the iron could carry out some biochemical reaction,” Shelnutt says. “They weren’t aware that the heme acted as part of the protein ‘machine’ and changed shape or even had shape. Many even believed it was flat [which it is outside the protein]. We’ve shown that that inside the protein the heme is three-dimensional, or nonplanar, and its shape corresponds to function.”
Further, he’s demonstrated that in many instances the heme’s shape changes as it performs its job.
“The main thing my discovery has done is provide a new way to look at heme proteins and what they do,” he says. “We’re revealing previously hidden influences of the protein on the heme structure.”
Shelnutt says that initially his findings were so startling that when he first tried to publish his results “no one believed me or would publish them.”
His observations finally appeared in a 1998 issue of Biophysical Journal. Since then, his research has captured the interest of the biochemical and biophysical communities. At a March meeting of the American Chemical Society in Anaheim, Calif. the two invited seminars he presented were standing-room-only.
Shelnutt’s discovery was made possible by a computer program developed about three years ago by his then postdoctoral student Walter Jentzen. The program, the only one of its kind, uses a mathematical procedure for characterizing the structure of hemes in terms of normal coordinates.
When heme proteins are put into a solution and the water slowly evaporates, they emerge in a crystalline form. Protein X-ray crystallographers then determine the positions of the atoms in these very large molecules and deposit this structural information in the Brookhaven Protein Data Bank. The structures can then be put into the computer program, which quantifies the heme’s nonplanar structure. Shelnutt also uses a light-scattering method, called resonance Raman spectroscopy, to detect the structure of proteins in solution.
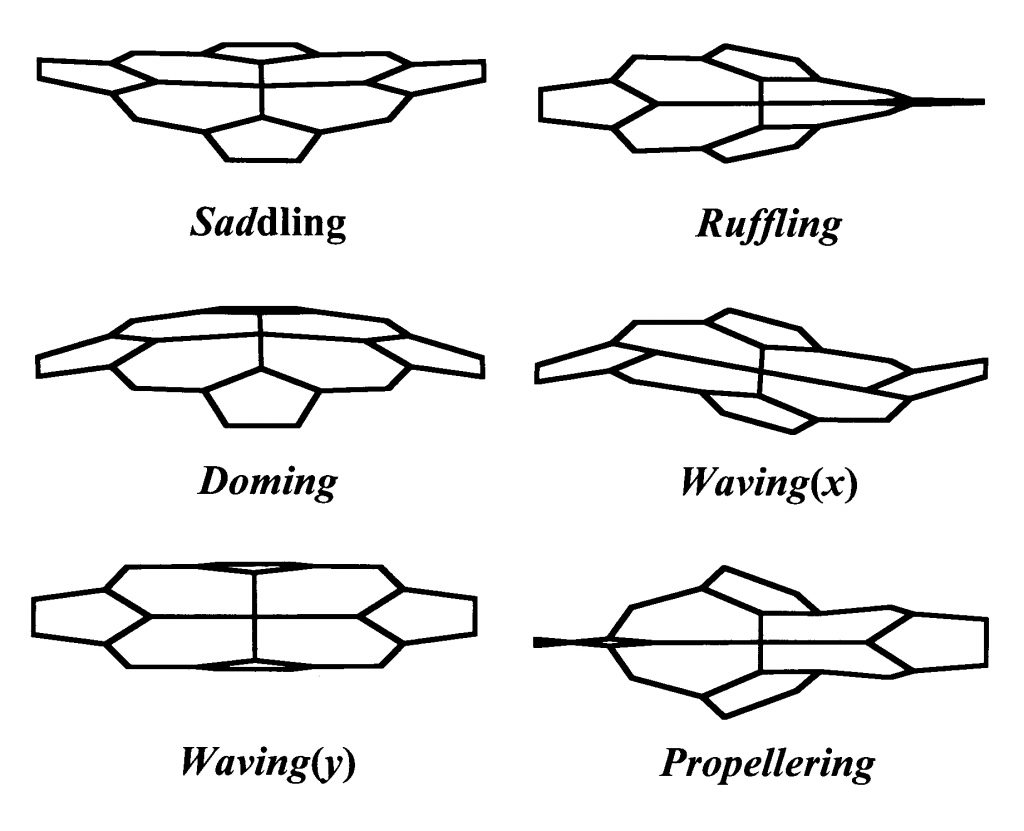
So far Shelnutt has used the program to study more than 400 existing protein crystal structures. And while he has identified some 70 different heme shape variations (called distortions), he has determined that only six are important for hemes in proteins — including saddling, ruffling, doming, propellering, and two types of waving. Each was named according to how the heme appears. Only a small amount of energy causes distortion for these six shapes.
He has also determined that distortions are specific to functions and can be found in proteins from both similar and different species that perform similar tasks, making certain shapes “conserved.”
“Conserved heme structures within the proteins tell us that the heme’s shape plays an important role in determining the function of the protein,” Shelnutt says. “We can see that different proteins may perform similar functions from the shape of the heme.”
He adds that some 800 hemes have been identified in the Protein Data Bank. However, before his research, “people didn’t realize that the shapes of the hemes were conserved, that is, the same shape is found in multiple proteins from different species.”
Another new concept that Shelnutt’s research has revealed is that many hemes change shape and go from one distortion to another as they perform their work. For example, when a certain protein binds with oxygen, the heme in the protein changes shape, losing its ruffling distortion. This causes the protein itself to change shape, turning off a biochemical reaction called nitrogen fixation. Then, when oxygen is no longer around, the heme ruffles once again, and nitrogen fixation is switched back on.
“Sometimes, though, the heme doesn’t change at all,” Shelnutt says. “It just depends on the job that needs to be done.”
Shelnutt’s interest in protein analysis and hemes dates back about ten years when he was researching the prospect of engineering molecules to, for example, change gaseous fuel to liquid fuel. Having a history of working with heme proteins at Bell Labs, he wondered if a protein could alter the heme’s shape and function as well.
Shelnutt believes that his discovery might lead to changes in the way diseases are treated in the future or for other scientific advances. In collaboration with a French scientist, he is already using his knowledge to develop new anti-inflammatory drugs.
Another possibility is to understand how hemes from another protein Shelnutt is working with convert sulfites or nitrites — common pollutants — into hydrogen sulfide or ammonia. Then, analogous arrangements of hemes could be chemically engineered, produced in batches, and released to clean up waste sites.
“The revelation that heme shape is correlated to a protein’s function is so new that we don’t yet know where this knowledge can take us,” Shelnutt says. “We have a new key to better understand life and now we have to figure out what to do with it.”
Sandia is a multiprogram DOE laboratory, operated by a subsidiary of Lockheed Martin Corp. With main facilities in Albuquerque, N.M., and Livermore, Calif., Sandia has major research and development responsibilities in national security, energy, and environmental technologies and economic competitiveness.