Download 300dpi JPEG image, ‘Gourley.jpg’, 1.2Mb (Media are welcome to download/publish this image with related news stories.)
ALBUQUERQUE, N.M. — A “smart scalpel” mechanism intended to detect the presence of cancer cells as a surgeon cuts away a tumor obscured by blood, muscle and fat has been developed in prototype by scientists at the U.S. Department of Energy’s Sandia National Laboratories.
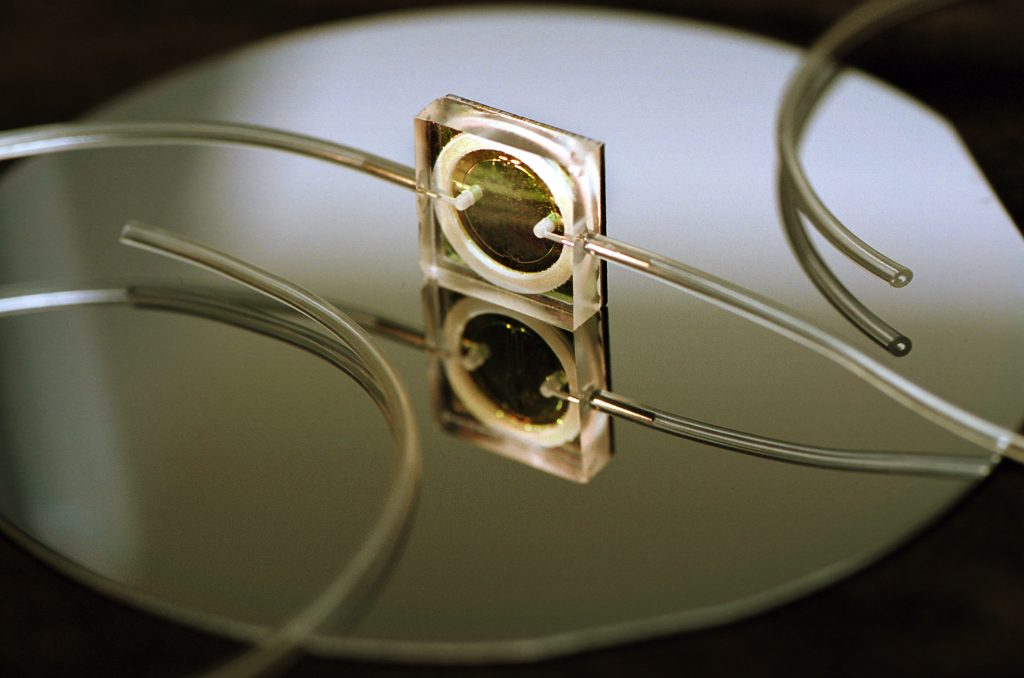
The dime-sized device, called a biological microcavity laser, should help surgeons more accurately cut away malignant growths while minimizing the amount of healthy tissue removed.
In effect, the patented device would tell a surgeon when to stop cutting.
“We can quickly identify a cell population that has abnormal protein content, as do tumor cells, by passing only a few hundred cells — a billionth of a liter — through our device,” says Paul Gourley, leader of the Sandia effort.
The device, more briefly referred to as a biocavity laser, has distinguished in the laboratory between cultured cells consisting of normal human brain cells called astrocytes and their malignant form, called glioblastomas, with excellent results.
The brain is a particularly critical place to know when enough tissue has been removed, says Gourley. Responses from other cells can be filtered out.
DOE has selected the work as best project of the year among its 28 US labs in a competition in its Basic Energy Sciences division.
A news conference sponsored by the American Physical Society will be held on Thursday, March 23 at the Minneapolis Convention Center to present latest findings on the device. A formal presentation will be made on March 24, also at the Minneapolis Convention Center, at the Society’s annual March meeting.
Download 300dpi JPEG image, ‘Scalpel.jpg’, 1.5Mb (Media are welcome to download/publish this image with related news stories.)
Can quickly distinguish cancerous from non-cancerous cells
According to Dr. Steve Skirboll, a member of the neurosurgery department at the University of New Mexico’s School of Medicine who is helping to determine the characteristics of the biocavity laser, “The device has great potential benefit, particularly if we continue to develop the nanotechnology at its base. We’re able to flow cells in real time, which no current device I’m aware of can do. We’re still looking at the basic science to nail down the major determining factor but the results are encouraging. We can show differences between tumor and non-tumor cells for the cancer we’re examining.”
Researchers didn’t believe it could be done. They were wrong.
Says Gourley, “People didn’t believe we could pump cells through a microlaser, make the cells part of the lasing process, and produce meaningful results. As it’s turned out, we can do all these things.”
The device works by incorporating blood cells into the lasing process, rather than shining a laser light like a spotlight upon the cell. A vertical microlaser beam enters individual cells as they are pushed by a micropump through tiny channels cut into the glass surface of the device. Because cancerous cells contain more protein than normal cells, their additional density changes (by refraction) the speed of the laser light passing through them. This change is registered as a difference in output frequency by a receiver and transmitted by optical fiber to a laptop computer a few feet from the instrument. An algorithm translates the data into a graph that, changing moment by moment, provides surgeons with easily read peaks and valleys that clearly depict when blood pumped from the incision has been cleared of cancerous cells.
In a surgical scalpel, an aspirator would vacuum fluid from the incision to the microcavity laser enclosed in the scalpel’s handle. Information would be transmitted from the scalpel to the computer by optical fiber.
Far quicker results
The microcavity laser is far quicker to produce results than flow cell cytometers –the standard instrument used to determine the presence of cancer cells obtained from an incision. Flow cell cytometers require cells to be stained with a dye in order to examine them. This lengthy process may take hours to alter the cells, and is of little immediate help to the patient, who has already been sewn back together.
The Sandia biocavity laser, based in part on semiconductor fabrication techniques, at an estimated cost from $10,000 to $50,000 is also far cheaper to build than a $100,000 (or more expensive) flow cytometry machine that may be desktop or benchtop size. The portable laser device has the potential to provide real-time analysis of up to 100,000 cells per second — a rate five times faster than other methods. It does not require — as do typical bench-top cytometers — a small room, highly trained operators, and a large laser.
DOE’s Office of Basic Energy Sciences and Sandia’s Laboratory-Directed Research and Development office, which supports discovery-oriented research, fund the materials research underlying this work.
Original disbelief
The device is an outgrowth of more than two decades of work at Sandia on compound semiconductor materials and microcavity laser structures. It could be said to have its roots 14 years ago when Sandia researchers succeeded — against much disbelief in the scientific community — in joining nanometer-thick layers of crystalline materials together to form a vertical cavity laser in the form of a single lattice. This achievement had been thought impossible, since the ultrashort dimensions of the laser’s active medium were not thought to permit laser operation. However, the sandwiching materials were so highly reflective that the device worked.
Achievement of these crystalline structures made it possible to routinely create tiny, very efficient lasers out of semiconductors in which nanometer-thick layers of gallium aluminum arsenide are sandwiched between nanometer-thick layers of gallium arsenide. Energizing the middle layer makes it emit photons, as would a crystal. The layers below and above it act as mirrors, reflecting emitted photons back and forth through the emitting material and amplifying the output in the classical process of a laser, though it all happens within horizontal spaces measured in nanometers.
In the biological microcavity laser, one microlaser beam is all that’s needed to intersect cells passing in a channel over the surface of the glass. More channels and more lasers can be added to increase the speed of the technique.
Recently published technical papers about the project have appeared in The Journal of Biomedical Microdevices and the Proceedings of the Biomedical Optics Society (SPIE).
Preliminary discussions are being held with biotech companies interested in commercializing the technique.
With further development, the microsensing device could also be deployed as an inexpensive, fast monitor of biological and chemical constituents of groundwater, waste fluids, or explosive chemicals. It is already able to detect other blood protein abnormalities, such as sickle cell anemia.
