
ALBUQUERQUE, N.M. — It was a small problem: a layer of water lying flat instead of slightly bumpy as it froze on a solid.
It became a larger problem when no one could explain why that might happen.
The slight difference between experimental results and established expectations might have meant nothing. But possibly it was signaling a basic scientific misunderstanding concerning the interaction of water with solids — an area of major industrial and scientific concern.
Water-surface interactions control the rate at which water passes through microscopic pores — a factor of increasing importance in micro- and nanotechnology. These interactions also affect the adhesion of materials in humid environments, catalytic chemical reactions, and condensation on dust particles in the upper atmosphere, to name a few.
Eventually, the problem ended up on the desk of theoretical physicist Peter Feibelman at the Department of Energy’s Sandia National Laboratories. His solution, which theorizes that water molecules dissociate near the surface rather than remain intact, is published in the Jan. 4 Science.
“This work makes the goal of understanding what happens when water contacts surfaces seem just a bit more achievable,” Feibelman said.
The problem
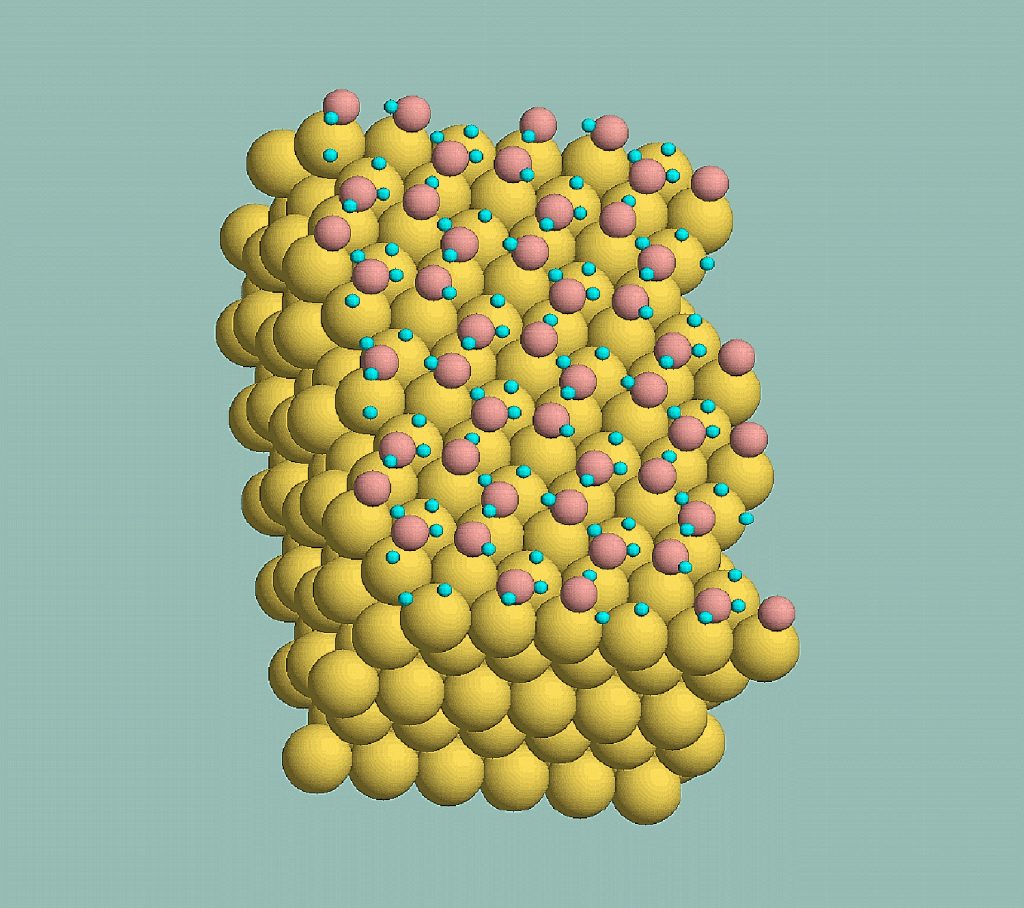
Several years ago Munich experimental physicists Georg Held and Dietrich Menzel found that the initial layer of water molecules didn’t lie the way they should on the precious metal ruthenium.
The researchers knew that ruthenium’s surface atoms pack tightly together in a hexagonal array. They also knew that water molecules of ice crystals do the same, in hexagons only marginally bigger than the metal’s. So the experimentalists expected that a frozen layer of water molecules would compress slightly and lie on the ruthenium with all the normal characteristics of a layer of ice.
But a small problem intruded from the third dimension: water molecules of ice always arrange themselves in puckered hexagons, with half the molecules higher and half lower.
Held and Menzel found no pucker.
Their layer of (heavy) water on ruthenium was almost perfectly flat.
Not only shouldn’t it be flat, it shouldn’t be
Feibelman saw an opportunity to use modern advances in theory to understand interfaces at the atomic level. “In the past, scientists could only summarize what goes on at surfaces by making assumptions that were embodied as boundary conditions,” he said. “The problem is that there was often no foundation for such simplifications.”
Feibelman hoped to use models faithful to nature at the atomic scale to interpret what was going on at the solid-water interface. He already had done significant work on the arrangements and movements of atoms on the surface of materials. At his desk, he reflected on how the chemistry of a solid surface might determine the arrangement of nearby water molecules.
Then he calculated the binding energy of the water molecules in the expected puckered structure versus their binding energy in pure ice. Feibelman reasoned that if ice is more favorable energetically, then water molecules will not want to spread out into a flat, essentially 2-dimensional layer on a ruthenium surface, but instead cluster together, forming a 3-D “ice cube.” And that — unfortunately — was just what he found. “I realized I was unable to explain why there is a 2-D layer at all,” he said, “to say nothing of why the layer was flat instead of puckered.”
Using observed facts rather than conventional assumptions
Then Feibelman thought, “If you take the experimental observation seriously — that all the oxygens are lying in same plane rather than in a puckered structure — then each oxygen atom is at about the same distance from a ruthenium atom.
“That means all the oxygens should bond to ruthenium atoms. But the only way this can happen is if the upper molecules of the expected puckered arrangement get rid of one of their deuteriums. Oxygen atoms that lose deuteriums will need to bind to something else, and ruthenium atoms are the obvious candidate.” (Note: the water in Held & Menzel’s experiments was ‘heavy water’ — deuterium replacing hydrogen — used because it produced electron diffraction patterns that were easier to analyze.)
Excited by this idea, Feibelman tried a calculation that assumed deuterium atoms broken off from water molecules also would find someplace else to bind on the metal surface.
His results then appeared to answer both the question of why a 2-D layer exists at all, and why the oxygen atoms lie in the same plane.
“Atoms as light as deuterium barely have any effect on electron diffraction,” said Feibelman. “Held and Menzel’s experiment by itself could only tell us where the oxygen atoms were in the heavy water layer, but not the deuteriums. Theory, however, can tell us where they lie, and this information leads to a quite new picture of how water wets to a metal. My results make it more than a little plausible that half the deuteriums separate from the water molecules where they originally were.”
Sandia Technical Contact:
Peter Feibleman, pjfeibe@sandia.gov (505) 844-6706
