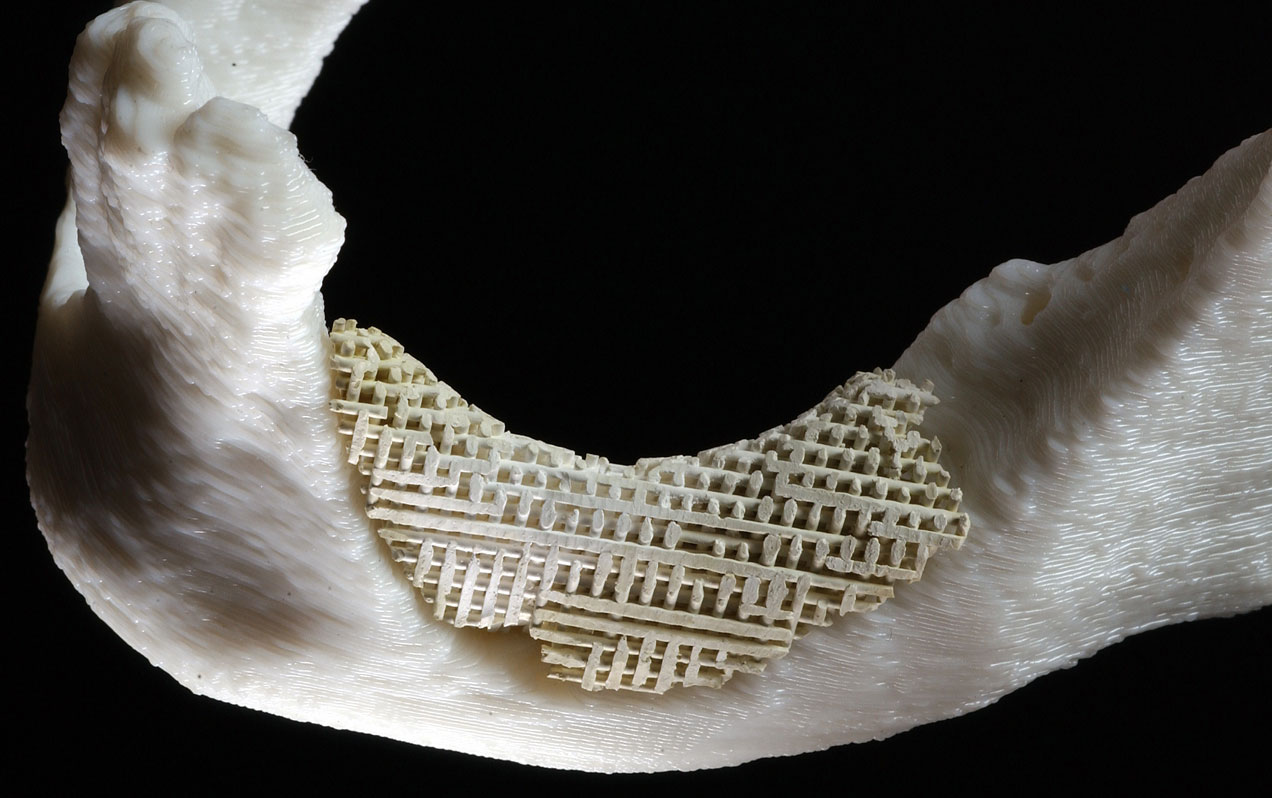
ALBUQUERQUE, N.M. — In an operating room in Carle Hospital in Urbana, Ill., on May 7, as scientists from the University of Illinois (UI) and Sandia National Laboratories watched, surgeon Michael Goldwasser fitted a highly unusual ceramic prosthetic device into the mouth of an elderly woman who had lost most of her teeth and along with it, much of the bone of her lower jaw.
The fitting operation was to determine whether the implant — created a thousand miles away at Sandia in Albuquerque — had been accurately designed, from its overall shape down to inclusion of a nerve groove.
“If it fit like a sock on a rooster, our method wouldn’t have worked,” Goldwasser said.
Observers said it fit like a glove.
If approved by the Food and Drug Administration for in vivo testing, the scaffoldlike structure — a layered mesh stronger than bone, yet porous — would substitute for a portion of the mandible, or lower jaw, until healthy, newly grown bone and blood vessels could weave their way through it like vines through a garden lattice. A patent for the implant is pending.
The ceramic scaffolding would reduce the pain, recovery time, and chances of infection of those needing bone replacements in the jaw, as well as skull, spine, or other bony areas. Other benefits include avoidance of longer surgeries, more predictability of outcome, and lower health care costs.
The device is built mainly of hydroxyapatite, a material already approved by FDA for bodily implants, so approval of the new device could be swift.
The woman was reportedly pleased to be part of an experiment that might benefit humanity, because the quality of fit would determine whether scientists and doctors using computer programs, modern communications, and machines a thousands miles from each other could produce a prosthetic device that would fit seamlessly in a patient’s sensitive mouth — or, for that matter, skull or spinal vertebrae — without the manufacturers ever seeing the patient.
But because scientists have studied the device’s strength and permeability only in vitro (in the lab), the woman then had to endure the standard method of bone replacement, which by comparison seems almost medieval. This involves cutting a several-square-inch piece of bone from her pelvis, which is then power-sawn and drilled into the correct shape in the operating room, a process that takes about an hour and leaves the patient to endure a healing pelvis as well as a healing mouth.
“Surgeons and patients would love to eliminate both the bone retrieval and implant preparation processes,” says Sandia scientist Joe Cesarano, whose team fashioned the new implant. “This test showed we can make artificial porous implants prior to surgery that will fit perfectly into the damaged region. The reconstructive procedure would then only require attaching the implant and closing the wound.”
A short course on bone implants
A surgeon uses the patient’s own bone to minimize rejection by the body. Harvesting bone, however, creates new problems, says Goldwasser. Not only is a new area of patient discomfort created but the operation requires more time and anesthetics. These raise the risks of complications in the operation and in healing. “We could use cadaver bones,” he says, “but then we face risks of rejection by the host and of possible transfer of disease.”
The body may also dispose of the foreign bone prematurely by absorbing it.
“What we want,” Goldwasser says, “is a method by which I can see a patient in Illinois, transmit X-ray information to someone who can make a substitute part that would have the porous properties that would allow bone to grow into it, yet be strong enough for normal function. Here, this would mean mastication and appearance.”
With the aid of UI bioengineering professor Russ Jamison and graduate and Sandia summer student Jennifer Dellinger, who were experimenting with the growth of bone across porous surfaces and needed a more regularly porous substrate than those found in nature, he learned of a device at Sandia that could do the job.
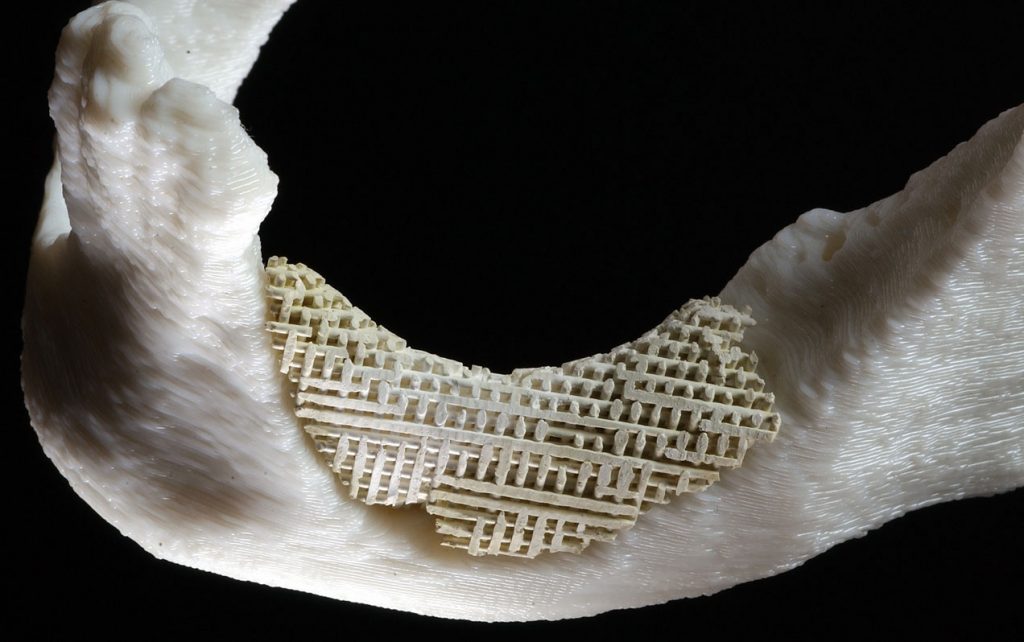
The Sandia device
The Sandia patented process called Robocasting, led by Cesarano, was conceived and built to fashion defense components out of ceramics in a process that permitted manufacture of specialty parts in a way no ordinary mold or machining procedure could achieve. Situated on a truck, it could make replacement parts on a battlefield, instead of carrying millions of parts onto a site. It is also being developed to form advanced catalyst supports that operate like a maze (rather than straight channels) to increase chemical reactivity.
Controlled by a computer program, the machine dispenses liquefied ceramic pastes, like toothpaste squeezed from a tube, to form shapes of varying complexity along a prearranged path.
To create the simulated bone scaffolding, the machine dispensed a hydroxyapatite mixture in a child’s Lincoln Log-like arrangement, in cross-laid slivers each about as thick and as far apart as the diameters of ten human hairs.
“Bone, blood vessels, and collagen love to grow into a structure with pores of that size [500 microns],” says Cesarano. “The material becomes a hard-tissue scaffold for promoting new bone growth.”
The trick in building it, he says, is that “the paste has to be strong enough as it’s being laid to set in place without under support.”
Sandian John Stuecker made the paste thicker than normal by increasing the interparticle attractive forces. The changed procedure took about six months to master.
Finally, the scaffolds are embedded in wax and machined to exactly the right shape without splintering the hydroxyapatite. The wax is subsequently removed. But what was that shape, and how was it to be determined?
The UIUC connection
In part, by the spark of an idea from Goldwasser and the diverse expertise linked together by Jamison.
“One by one, we linked together — even if only electronically at first — the people whom I knew who could bring talent, skill, and passion to the project. None of us working independently could have accomplished the results we have,” he says.
Jamison involved computer technologists and designers Ben Grosser and Janet Sinn-Hanlon at UI’s Beckman Institute to encode CAT scan results into a computer program that could be shipped electronically to Sandia, where Michael Saavedra and David Gill created an interface to machine the final shape.
Complicating the process was that while a CAT scan could accurately delineate the diseased shape of an existing bone, it could not show what wasn’t there: the exact dimensions of what the bone would have looked like, were it healthy. This required the potentially expensive presence of the surgeon Goldwasser working with the computer programmers to create the dimensions of what should be there but wasn’t.
“Eventually, if it could be done electronically, it may be a very simple thing and cost-effective,” he said.
“There is nothing inherently expensive about either the materials or the process,” said Cesarano.
Using a CAD/CAM method where a surgeon need only sketch the shape needed, a piece might quickly and inexpensively take shape at a remote site.
“We’ll see if the clinician, the bioresearcher, and the engineer can come up with a method to implement it,” Goldwasser said.