Download 150dpi JPEG image, ‘self_org.jpg’, 270K
LIVERMORE, Calif. — Get organized!
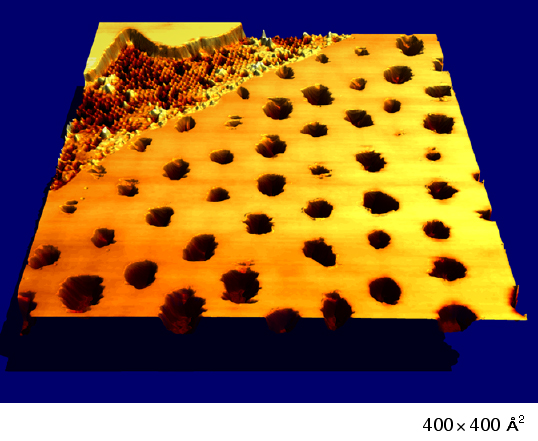
When this process takes place on a single-atom-thick film of silver sprinkled with sulfur, a lacework pattern emerges with surprising precision.
At the Department of Energy’s Sandia National Laboratories, post-doctoral fellow Karsten Pohl has been carefully examining this phenomenon. He says characterizing what drives this process could enable new generations of revolutionary nano-structures whose manufacture rivals the most advanced patterning techniques.
In the lattice-work studied, sulfur atoms pierce the silver “in a spectacular example of self-organization,” adds Norm Bartelt, a theoretical physicist who statistically analyzed the process. “The individual holes the sulfur digs line up into a very perfect lattice.”
Each hole is almost 25 times further apart than the field of force exerted by individual atoms. How these distant features interact to form surface patterns was intriguing and mysterious for the research team, which included Sandians Maria Bartelt and Juan de la Figuera. The researchers say the answer might allow creation of such devices as computers based on islands of quantum dots.
Collaborators at Sandia were the first to combine statistical analysis and experimental observations to probe the forces governing this behavior.
They used a scanning tunneling microscope at Sandia that has such good resolution that it was possible to watch clusters of atoms move as the seconds ticked by. The silver film is spread out on the ruthenium substrate into a layer one silver atom deep, which offers a preferred low-energy state. The added sulfur also wants to rest directly on top of the ruthenium to minimize energy.
As they jostled for position during self-organization, groups of sulfur atoms displaced silver atoms and formed islands of sulfur embedded in the silver sheet. The flat ruthenium underneath started to “cup” slightly under the strain as the silver sheets were distorted to accommodate the sulfur islands. These distortions caused the sulfur islands to repel each other and organize into an ordered pattern.
The forces of this bulk elastic distortion are very weak — about 10,000 times less than the electrical forces that operate at close range between atoms. But the findings still hold promise that by controlling the substrate, the pattern-forming interactions can be controlled and even tuned to create specific patterns.
Patterning at such small scales is extremely difficult, so this propensity for self-organization could present an ultimate approach to making ordered arrangements.
The team has received internal Sandia research funding to see how general the phenomenon is. For instance, it might be possible to put a different metal in the lattice-work “holes” to make templates for a sensor, says Sandia physicist Bob Hwang.
He and visiting collaborator Jan Hrbek of Brookhaven National Laboratory, an expert on sulfur, first examined this system to better understand how sulfur poisons catalytic converters (that clean vehicle emissions) by changing surface structures — a corrosion problem of long interest. However, they did not expect the corrosion to exhibit such an ordered pattern.
For more information about this research, see Jan. 21, 1999 issue of the journal Nature (pp. 238-241, “Identifying the forces responsible for self-organization of nanostructures at crystal surfaces,” by K. Pohl, M.C. Bartelt, J. de la Figuera, N.C. Bartelt, J. Hrbek and R.Q. Hwang.) For a copy of the journal article, call Nature at (202) 737-2355, or Barbara Troen at Sandia/California Public Affairs at 1 (800) 472-6342, extension 4-3135.
