
ALBUQUERQUE, N.M. — A coating that allows miniature sensors to detect dangerous, even lethal, air- or water-borne molecules much more quickly has been created in a joint project of Sandia National Laboratories and the University of New Mexico. The achievement is reported in the current issue of the journal Nature.
The film-like coating — less than one micron thick — barely increases the size of the sensor. But the material’s extreme porosity increases the sensor’s surface area, and therefore sensitivity, by a factor of about 500, says Sandia principal investigator Jeff Brinker.
“Imagine tasting something sour, and then 500 times more so,” says Brinker.
The film’s sensitivity could help combat terrorism, lead to smaller yet more accurate sensors for environmental monitoring, and benefit oil and pharmaceutical companies, which use molecular separations to produce grades of gasoline and a variety of drugs, says Brinker.
Funding was received through the Department of Energy’s (DOE) Basic Energy Sciences program, and the UNM/National Science Foundation Center for Microengineered Materials. Sandia is a DOE multi-program national security laboratory.
The extremely high surface area of the film can be modified to detect chemical weapons like Sarin, used in the terrorist attack in the Tokyo subway. Under laboratory conditions, the film applied to a sensor detected 200 parts per billion of a Sarin simulant. A human being can survive approximately 50 minutes at those concentrations, says Brinker.
How does it do that?
The material, honeycombed by tiny tunnels of precise size, acts as a membrane to separate molecules of differing dimensions.
The film functions similarly to zeolites — porous crystalline pebbles used by the oil industry to separate out molecules of different sizes. Honeycombed by tiny tunnels, zeolites filter out molecules too big to pass through them, trap mid-sized ones, and allow passage to smaller ones.
Unlike some zeolites, the Sandia coating — a lightweight gel — must be created artificially. However, because the gel’s molecules self-arrange themselves into a kind of molecular rug, no machinery of assemblage is necessary. Also, while a zeolite’s pores never exceed a dimension of 13 angstroms, the pore sizes of the Sandia coating are controllable by scientists and can be made as large as 100 angstroms to accommodate a variety of molecules.
While such artificial coatings long have been desired, scientists could not produce an interior network of tiny tunnels that allowed large numbers of molecules to enter the surface and exit the base of the film, rather than be halted at dead ends. Another problem — not solved until development of the coating — was making it seamless, so that molecules intended for inspection could not evade the sieve by flowing through cracks in it.
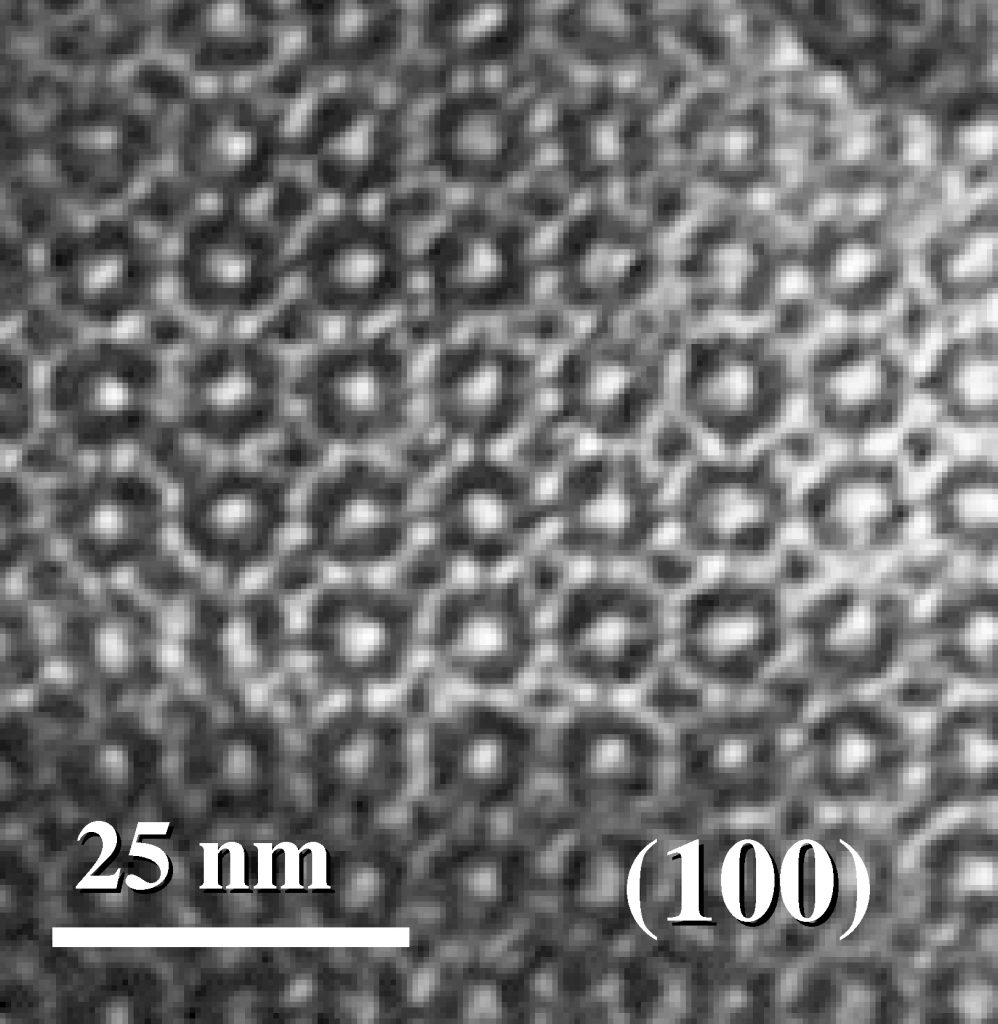
More flawless than grandma’s knitted blanket
In the Sandia method, the gel is made seamless by continually removing a substrate from a liquid bath. The gel — a nanocomposite — forms seamlessly as water and alcohol evaporate.
Earlier methods, which allowed the gel to dry on sections of substrates, formed fractured borders that made the gel useless as a sieve.
Seen by transmission electron microscope, the coating’s molecular structure resembles a grandma’s endless knitted blanket with never a stitch missed.
The continuous formation of a kind of cubic arrangement of molecules guarantees that a sizable number of pores accessible at the film’s surface will pass completely through it.
“Everything depends on the initial surfactant [surface-acting molecular] concentration we choose,” says Brinker.
Circling the wagons
The materials of the gel self-assemble due to the two-sided organic surfactant molecules.
One side is hydrophobic (hates water) and the other hydrophilic (loves it). In a solution of water and silica, small groups of organic molecules perform tiny versions of circling the wagon trains, arranging their water-loving ends to face outward in wheel-like shapes. The circles form an ordered array. The water-phobic ends remain within the circles, somewhat like spokes and hub. Silica in solution, attracted by the circular rims, surrounds them like tires encircling wheels. After this self-assembly process, the organics are removed by heating (pyrolysis), leaving an inorganic silica fossil with a periodic arrangement of pores where the organics used to be.
The work is a collaboration between Brinker, Sandia researcher Celeste Drewien, Mark Anderson (formerly at Sandia, now at 3M), UNM students Yungfeng Lu and Rahul Ganguli, with Professors Bruce Dunn and Jeff Zink at the University of California at Los Angeles.
Sandia is a multiprogram DOE laboratory, operated by a subsidiary of Lockheed Martin Corp. With main facilities in Albuquerque, N.M., and Livermore, Calif., Sandia has major research and development responsibilities in national security, energy, and environmental technologies and economic competitiveness.
