ALBUQUERQUE, N.M. — A benchtop version of the world’s smallest battery — its anode a single nanowire one seven-thousandth the thickness of a human hair — has been created by a team led by Sandia National Laboratories researcher Jianyu Huang.
To better study the anode’s characteristics, the tiny rechargeable, lithium-based battery was formed inside a transmission electron microscope (TEM) at the Center for Integrated Nanotechnologies (CINT), a Department of Energy research facility jointly operated by Sandia and Los Alamos national laboratories.
Says Huang of the work, reported in the Dec. 10 issue of the journal Science, “This experiment enables us to study the charging and discharging of a battery in real time and at atomic scale resolution, thus enlarging our understanding of the fundamental mechanisms by which batteries work.”
Because nanowire-based materials in lithium ion batteries offer the potential for significant improvements in power and energy density over bulk electrodes, more stringent investigations of their operating properties should improve new generations of plug-in hybrid electric vehicles, laptops and cell phones.
“What motivated our work,” says Huang, “is that lithium ion batteries [LIB] have very important applications, but the low energy and power densities of current LIBs cannot meet the demand. To improve performance, we wanted to understand LIBs from the bottom up, and we thought in-situ TEM could bring new insights to the problem.”
Battery research groups do use nanomaterials as anodes, but in bulk rather than individually — a process, Huang says, that resembles “looking at a forest and trying to understand the behavior of an individual tree.”
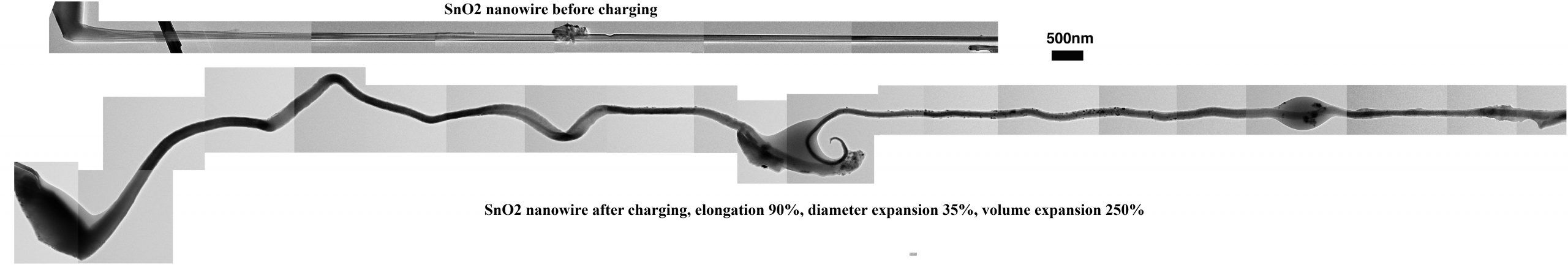
The tiny battery created by Huang and co-workers consists of a single tin oxide nanowire anode 100 nanometers in diameter and 10 micrometers long, a bulk lithium cobalt oxide cathode three millimeters long, and an ionic liquid electrolyte. The device offers the ability to directly observe change in atomic structure during charging and discharging of the individual “trees.”
An unexpected find of the researchers was that the tin oxide nanowire rod nearly doubles in length during charging — far more than its diameter increases — a fact that could help avoid short circuits that may shorten battery life. “Manufacturers should take account of this elongation in their battery design,” Huang said. (The common belief of workers in the field has been that batteries swell across their diameter, not longitudinally.)
Huang’s group found this flaw by following the progression of the lithium ions as they travel along the nanowire and create what researchers christened the “Medusa front” — an area where high density of mobile dislocations cause the nanowire to bend and wiggle as the front progresses. The web of dislocations is caused by lithium penetration of the crystalline lattice. “These observations prove that nanowires can sustain large stress (>10 GPa) induced by lithiation without breaking, indicating that nanowires are very good candidates for battery electrodes,” said Huang.
“Our observations — which initially surprised us — tell battery researchers how these dislocations are generated, how they evolve during charging, and offer guidance in how to mitigate them,” Huang said. “This is the closest view to what’s happening during charging of a battery that researcher have achieved so far.”
Lithiation-induced volume expansion, plasticity and pulverization of electrode materials are the major mechanical defects that plague the performance and lifetime of high-capacity anodes in lithium-ion batteries, Huang said. “So our observations of structural kinetics and amorphization [the change from normal crystalline structure] have important implications for high-energy battery design and in mitigating battery failure.”
The electronic noise level generated from the researchers’ measurement system was too high to read electrical currents, but Sandia co-author John Sullivan estimated a current level of a picoampere flowing in the nanowire during charging and discharging. The nanowire was charged to a potential of about 3.5 volts, Huang said.
A picoampere is a millionth of a microampere. A microampere is a millionth of an ampere.
The reason that atomic-scale examination of the charging and discharging process of a single nanowire had not been possible was because the high vacuum in a TEM made it difficult to use a liquid electrolyte. Part of the Huang group’s achievement was to demonstrate that a low-vapor-pressure ionic liquid — essentially, molten salt —could function in the vacuum environment.
Although the work was carried out using tin oxide (SnO2) nanowires, the experiments can be extended to other materials systems, either for cathode or anode studies, Huang said.
“The methodology that we developed should stimulate extensive real-time studies of the microscopic processes in batteries and lead to a more complete understanding of the mechanisms governing battery performance and reliability,” he said. “Our experiments also lay a foundation for in-situ studies of electrochemical reactions, and will have broad impact in energy storage, corrosion, electrodeposition and general chemical synthesis research field.”
Other researchers contributing to this work include Xiao Hua Liu, Nicholas Hudak, Arunkumar Subramanian and Hong You Fan, all of Sandia; Li Zhong, Scott Mao and Li Qiang Zhang of the University of Pittsburgh; Chong Min Wang and Wu Xu of Pacific Northwest National Laboratory; and Liang Qi, Akihiro Kushima and Ju Li of the University of Pennsylvania.
Funding came from Sandia’s Laboratory Directed Research and Development Office and the Department of Energy’s Office of Science through the Center for Integrated Nanotechnologies and the Energy Frontier Research Centers program.