
Download 300dpi JPEG image, ‘shelnutt-song.jpg’, 448K (Media are welcome to download/publish this image with related news stories.)
ALBUQUERQUE, N.M. — Researchers from the Department of Energy’s Sandia National Laboratories and the University of New Mexico have developed a new way of mimicking photosynthetic proteins to manipulate platinum at the nanoscale. The method has the potential of changing the metal’s properties and benefiting emerging technologies.
“While we are in the early stages of research, we see the possibility of manipulating the nanoscale structure of platinum so that we can have control over the size, porosity, composition, surface species, solubility, stability, and other functional properties of these metal nanostructures,” says John Shelnutt, the Sandia scientist leading the research effort. “Such control means that the redesigned platinum could be used in many new applications, including catalysis, sensors, and optoelectronic and magnetic devices.”
He adds that while research groups have reported a few platinum nanostructures — including nanoparticles, nanowires, nanosheets, and others — the addition of new types of nanostructures is “highly desirable and potentially technologically important.”
Working with Shelnutt in the research are Frank van Swol from Sandia, UNM graduate student Yujiang Song, and Eulalia Pereira from the University of Porto in Portugal.
The new method of manipulating platinum was detailed in a paper in the Journal of American Chemical Society published in late December.
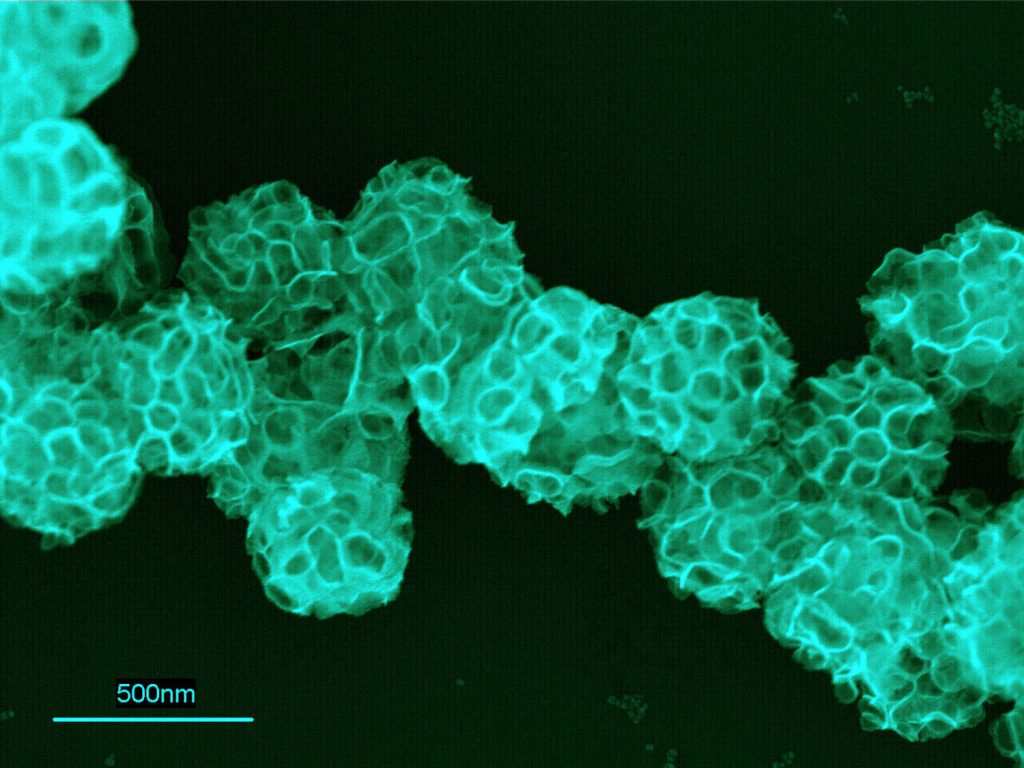
Download 300dpi JPEG image, ‘foamball.jpg’, 256K (Media are welcome to download/publish this image with related news stories.)
The idea for the technique is similar to photosynthesis, in which plants use the energy from sunlight to produce sugar. But instead of manufacturing sugar, the new method changes a platinum ion to the neutral metal atoms. The photosynthetic protein mimicks this repeatedly, allowing metal to be deposited as desired at the nanoscale.
The method involves putting porphyrins — the active part of photosynthetic proteins — along with the platinum salt in an aqueous solution of ascorbic acid at room temperature. The porphyrins are placed in specific locations in the solution where it is intended that metal should be deposited. For example, the porphyrins may be confined to micelles or liposomes. Micelles are spherical assemblies of detergent molecules in which the heads are exposed to the water and the tails stick together in the interior. Liposomes are similar structures but they are larger and have water on the inside and outside separated by a closed membrane — sort of like a cell. The membrane is composed of two layers of detergent molecules, with the heads on the inner and outer surface facing the water and the tails forming the interior of the membrane.
When light is shined on the porphyrins located in these detergent structures, the porphyrins excite, becoming catalysts for platinum reduction and deposition. As this occurs, the metal grows onto the surfaces of the surfactant structures as a thin sheet or in other ways. In the case of micelles, the platinum grows into balls that look like the common toy “Koosh™” ball. The ball size can be controlled by the amount of porphyrins and platinum in the solution, the amount of light illuminating the solution, and the amount of time the light is on.
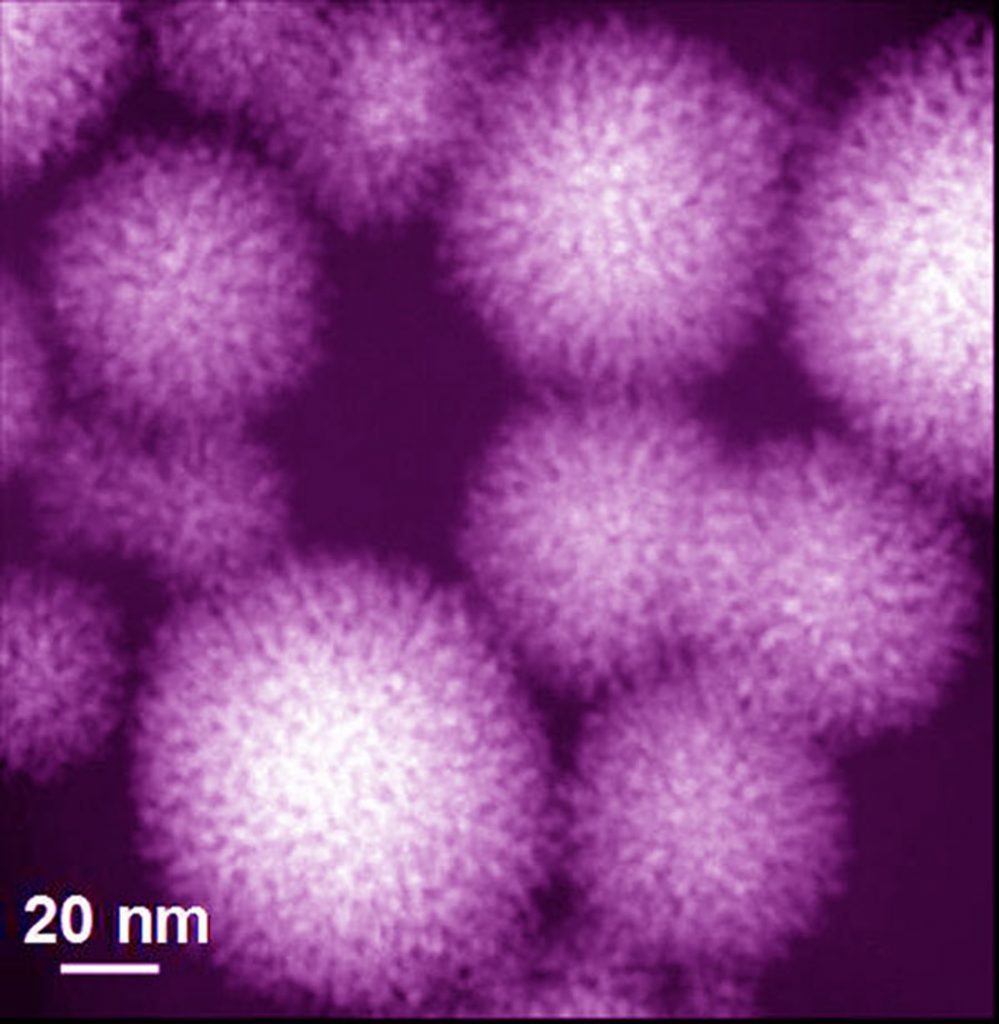
Download 300dpi JPEG image, ‘nanokoosh.jpg’, 268K (Media are welcome to download/publish this image with related news stories.)
For the metals platinum and palladium that form these nanostructures, it is enough for the porphyrin molecule to grow only a small metal “seed” particle composed of about 500 atoms. When it reaches this size, the seed starts to catalyze its own rapid growth (by oxidation of ascorbic acid), budding off arms in all directions and creating the Koosh-ball-like nanostructures. The porphyrin provides a convenient method of making these seeds at the location and time desired, leading to a uniform and selectable nanostructure size.
The platinum nanostructures take on a different form when they are prepared under different conditions. When the porphyrin is in a micelle, the platinum nanostructures produced look like Koosh balls. When the porphyrin is in the bilayer membrane of a liposome, the platinum grows in 2-nanometer thick sheet or platinum lace on the outer surface of the membrane, giving circular sheets — sort of like two-dimensional Koosh balls.
Under solution conditions for which the liposomes aggregate, growth can occur along the interfaces of the liposomes to give platinum foamlike materials and foam nanoballs. The type of nanostructure is mainly determined by the type of surfactant assembly upon which the platinum grows and the extent of growth from the individual seed nanoparticles.
Since the porphyrin remains attached to the platinum nanostructure and active in the presence of light, it can also perform other functions besides growing itself. For example when illuminated with light, the platinum nanostructure evolves hydrogen from water. This reaction is similar to one of interest to car manufacturers looking to new ways to build automobiles powered by hydrogen fuel cells.
Shelnutt says that in addition to structuring the platinum, the process also happens very fast. A few minutes in light will create many seeds, which then grow into the mature nanostructures in tens of minutes. And the process is easy to do.
“It’s so simple it’s amazing,” Shelnutt says.
