Download 300dpi JPEG image, ‘Nenoff_SOMS.jpg’, 1.3MB (Media are welcome to download/publish this image with related news stories.)
ALBUQUERQUE, N.M. — Researchers studying ways to capture radioactive chemicals swimming in a sea of hazardous waste have created a new class of molecular cages that, like lobster traps, let certain species in while keeping others out.
The new microporous materials, named Sandia Octahedral Molecular Sieves (SOMS) by their discoverers at Sandia National Laboratories, could be useful in microelectronics fabrication and other industries where purification of, or extraction from, liquid process or waste streams is a significant or costly problem.
They also could help capture for reuse a variety of valuable materials (such as chromium, cobalt, and nickel) from industrial effluents.
Sandia developed the SOMS in collaboration with researchers at the University of California – Davis (UC Davis), Pacific Northwest National Laboratory (PNNL), the University of Michigan, the State University of New York – Stony Brook (SUNY), and Lawrence Livermore National Laboratory (LLNL).
The work is sponsored by the Department of Energy’s Environmental Management Science Program, which funds projects that reduce long-term DOE-site cleanup costs.
Picky ion catchers
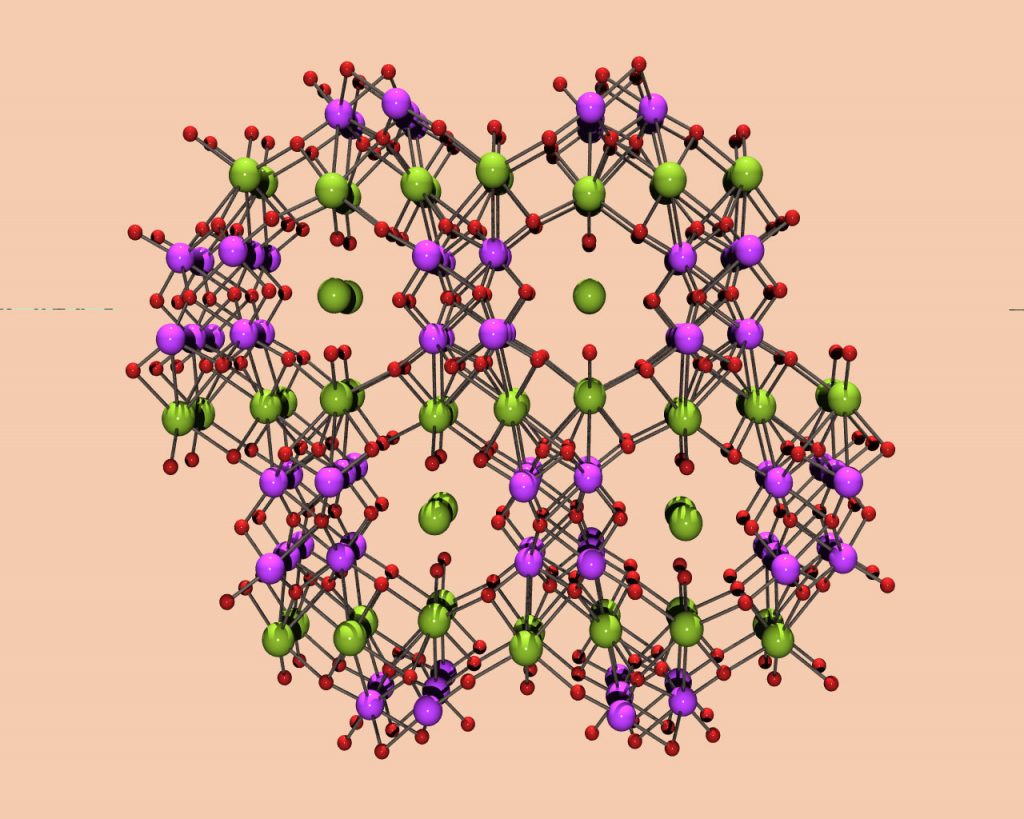
Chemically, a SOMS is a tiny sponge that sucks up divalent cations (atom groups with a +2 charge) into its microscopic pores and snares them at negatively charged bonding sites that have been vacated by ions with weaker charges — a process called ion exchange. (Home water softeners use ion exchangers to remove iron from tap water.)
Download 72dpi JPEG image, ‘micrograph.jpg’, 20K (Media are welcome to download/publish this image with related news stories.)
The SOMS are picky about which ions they capture because the sizes of openings on their crystalline surfaces can be adjusted precisely by altering the recipes followed to make them. By varying these openings from 4 to 15 angstroms (an angstrom is one ten-millionth of a millimeter), the researchers are able to select the sizes of ions or molecules that can get into the pores, and which can’t.
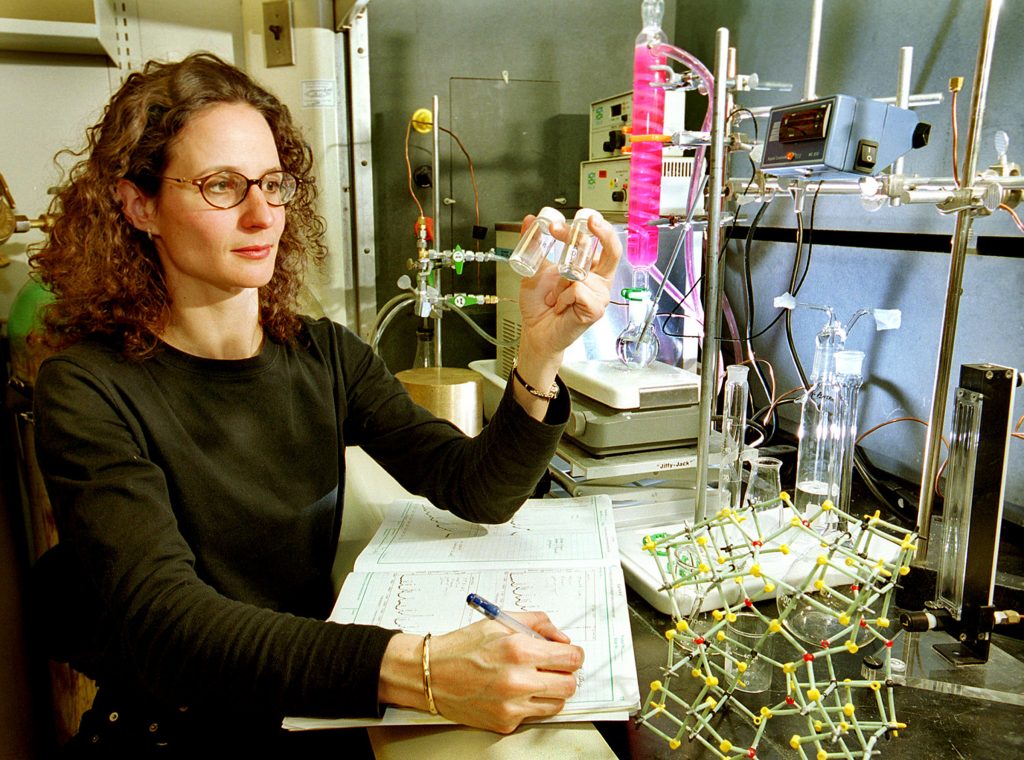
“Not only are SOMS fascinating as a new material,” says Sandia principal investigator Tina M. Nenoff, “they possess many unique properties that are useful in waste cleanup and industrial processing.”
The new SOMS are extremely selective for strontium-90, for instance, one of the two most prevalent radioactive constituents of liquid hazardous waste inside the 177 underground storage tanks at DOE’s Hanford, Wash., environmental remediation site.
In lab tests the SOMS trapped 99.8 percent of strontium-90 ions in parts-per-million concentrations from solutions containing chemically similar and highly abundant sodium ions.
“We can tune the pore size and the chemistry of the framework on the nano scale so the SOMS materials capture cations on the bulk scale very selectively and efficiently, and in all types of environments,” says Nenoff.
An unexpected bonus property
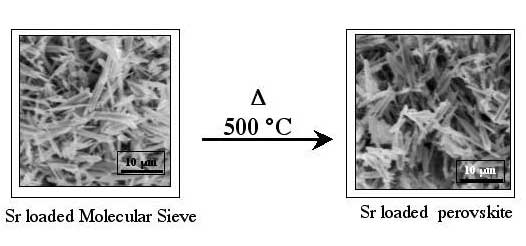
When heated to about 500C°, the SOMS collapse into a dense glass-like material called a perovskite, its shrunken pores locking the cations tightly into its crystalline structure. Bricks made from the densified SOMS are impervious to leaching and stable against high pH, radiation, and heat, which might make them ideal for disposal.
Download 72dpi JPEG image, ‘SOMS1.jpg’, 176K (Media are welcome to download/publish this image with related news stories.)
“This unique, unexpected property gives SOMS the added bonus of being ready for a waste repository or landfill after only minimal processing,” she says.
Liquid waste or processing solutions could be pumped through columns containing the SOMS, she says. When the SOMS became fully saturated with the desired cations, they could be densified and disposed of safely.
Technically SOMS are sodium niobium oxide with transition metals such as titanium or zirconium added to give the SOMS their microporosity and ion exchange properties.
Sandia already is studying ways to use SOMS to extract and reuse valuable cobalt from copper-mine electro-refinement waste streams.
Other resources:
Sandia’s Waste Legacy Program:
http://www.nwer.sandia.gov/wlp
DOE underground storage tank:
http://www.hanford.gov
http://www.pnl.gov/tfa
DOE’s Environmental Management Science Program:
http://emsp.em.doe.gov
SOMS development project:
http://emsp.em.doe.gov/portfolio/AbstractDetails.asp?trackID=495
Related Sandia news releases: