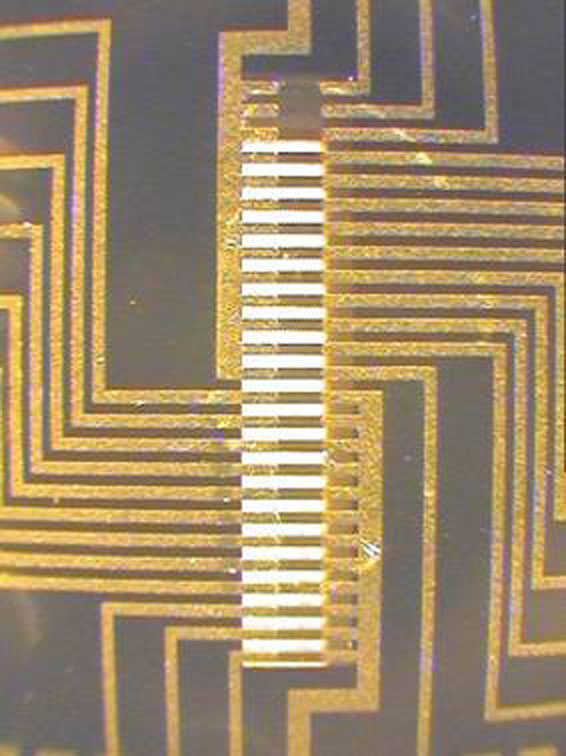
ALBUQUERQUE, N.M. — A microdevice whose business end looks like the gold-coated spine of a very tiny mouse, with each “vertebrae” line separated from the next by about a third the width of a human hair, has been demonstrated to easily collect and release proteins in aqueous solution in less than a second.
The device, reported in the current issue (July 18) of the journal Science, separates proteins from solution and from each other by electrically heating the tiny metal lines to alter surface properties, say Sandia National Laboratories researchers Dale Huber and Bruce Bunker. “We capture and release on command very quickly and from very definite locations,” says Huber of their research group’s ability to send current to selected heat lines, mimicking electrically the chemical separation methods of industry-standard chromatographs.
The device could fit easily into the hand-held sensors familiarly referred to as labs-on-a-chip, and aid in detecting terrorist attacks by near-instantaneously concentrating classes of suspect proteins for immediate analysis.
“Our methods make proteins very obedient,” says Huber. “They hang on or let go as we tell them, and they don’t denature [that is, they stay healthy] even after 24 hours.” The proteins are thus available for more extensive analysis than conventional separation methods ordinarily permit.
The device, for which Sandia has applied for a patent, works by sending minute currents of electricity for microseconds through the gold crosspieces called heat lines to warm a four-nanometer-thick polymer film. The film, called poly(NIPAM), responds to heat by changing from a hydrophilic (water-loving) to a hydrophobic (water-hating) state. The water-hating state makes it easy for the film to adsorb proteins passing over it in an aqueous solution, while the cooler hydrophilic state means the proteins will be outcompeted by water molecules and be released in a natural cleansing action.
Furthermore, because smaller proteins adsorb faster, a brief pulse of electric current is all that’s needed to separate them from the solution. The solution can then be disposed of, leaving only the small proteins. If large proteins are of interest, the runoff becomes valuable and the smaller proteins can then be disposed of by later letting the heating current die down. The small proteins release and can be disposed of by draining this solution. There is no fouling.
Lengthier application of current shows that because of their larger surface areas, larger proteins tend to displace the smaller, first-arriving proteins and attach to the polymer. Draining the solution then removes the small proteins. Thus the device acts not only as a fast, low-power preconcentrator of proteins, it can be used to change the ratio of large to small proteins as desired for easier analysis in specific applications.
For further microanalysis, Bunker says, “You could envision different streams perpendicularly across our fluid-carrying channel. We could release across any given channel, then further separate proteins based on charge or size.”
A spin-off application that seems possible to the research team would be to use the method to grab antigens in saliva or blood serum that would indicate a disease in progress. “Think of the use for GI Joe in the field,” says Bunker. “We’ll insert antibodies specific to particular diseases. Turn on the battery, raise the heat, and trap the antibodies to create a film. Then see if this film interacts with a blood serum or saliva sample. No? Turn off the heat, release the antibody, enter another antibody, and retry.”
Another difference between the new method and standard chromatography columns is that the latter use the same separation materials to get the same results every time. The Sandia approach enables researchers to program changes into the characteristics of the “column” by varying the temperature of the heat lines, as well as the length of time they are hot, along the channel through which the protein-carrying liquid passes. This enables researchers to vary the final spacing between protein classes of different size or weight to provide clearer outcomes.
“Also,” says Bunker, “we can work with smaller volume and very small quantities of protein.”
Finally, he says, “It’s also a good antifouling system. Proteins stick only where we tell them to.”
Currently, the researchers are working at the proof-of-concept phase, and have shown that proteins adhere, release, and displace one another on squares of heated poly(NIPAM) — formally, poly(N-isopropyl acrylamide). One goal of the research is to have a 3-D tube packed with coated micropebbles that would increase sensitivity by creating a 1,000-square-meter-per-gram surface area to analyze larger volumes of intake material. This could be integrated onto a microchip and heated with a larger resistive heater, or by an infrared laser.
While the device is envisioned to function near the front-end of an analysis unit, a still-prior device would be needed to provide proteins, either by opening cells to make their proteins available for analysis, providing fluids from humans, or by collecting protein from the environment.
“Currently, we’re working with a group at Sandia/California led by Bryan Kirby to make the technology useful by building it into their integrated platform,” Huber says.
The work is funded by DOE’s Division of Materials Sciences and Engineering, Office of Basic Energy Sciences, and by a Laboratory-Directed Research and Development grand challenge for Molecularly Integrated Microsystems.

