
Download 300dpi JPEG image, ‘uranium.jpg’, 592K (Media are welcome to download/publish this image with related news stories.)
ALBUQUERQUE, N.M. — An innovative “natural” alternative to cleaning up uranium-contaminated sites is being studied by scientists at the Department of Energy’s Sandia National Laboratories as a way to replace costly and sometimes ineffective traditional techniques.
Called natural attenuation, the method relies on a naturally occurring process that adsorbs soluble uranium and metals onto a mineral surface. As the coatings age and recrystallize, the uranium eventually becomes encased in the mineral and cannot escape into groundwater or soil.
“This is an innovative approach to uranium cleanup,” says Sandia scientist Malcolm Siegel who has been working with colleague Charles Bryan to characterize the process for three years. “Natural attenuation has become an accepted method for cleaning up soils and groundwaters contaminated with toxins that can be eliminated through biological processes — where microorganisms break down organic contaminants. Here, instead, the uranium becomes strongly bound to the mineral naturally.”
Such processes are of interest to those who want to clean up sites contaminated by uranium or heavy metals in as natural and inexpensive a way as possible. Siegel says traditional methods of cleanup, called active remediation, frequently involve “suck, muck, and truck” — digging up waste and physically removing it — and “pump and treat” — pumping contaminated water from the ground, treating it, and returning it. These techniques are generally costly and time-consuming.
Promises Cleanup savings
Costs of cleaning up U.S. hazardous waste sites, including uranium-contaminated sites, over the next three decades have been estimated at between $373 billion and $1.694 trillion. If natural attenuation is used instead in appropriate situations, the expense could be significantly reduced.
The Department of Energy (DOE) is seeking cheaper, better, faster ways of cleaning up sites and sees natural attenuation using iron oxyhydroxides as one possible method of reaching this goal. The Environmental Protection Agency (EPA) also considers it a valid remediation action.
The Sandia uranium natural attenuation research is part of a larger multi-agency, multi-lab endeavor. The project was funded in part over the past three years by Sandia’s Laboratory Directed Research and Development (LDRD) program. Project research focused on heavy metals and radionuclides. Natural attenuation of radionuclides, heavy metals, and organics is also the subject of on-going work at Sandia funded by the EPA, Nuclear Regulatory Commission, and DOE.
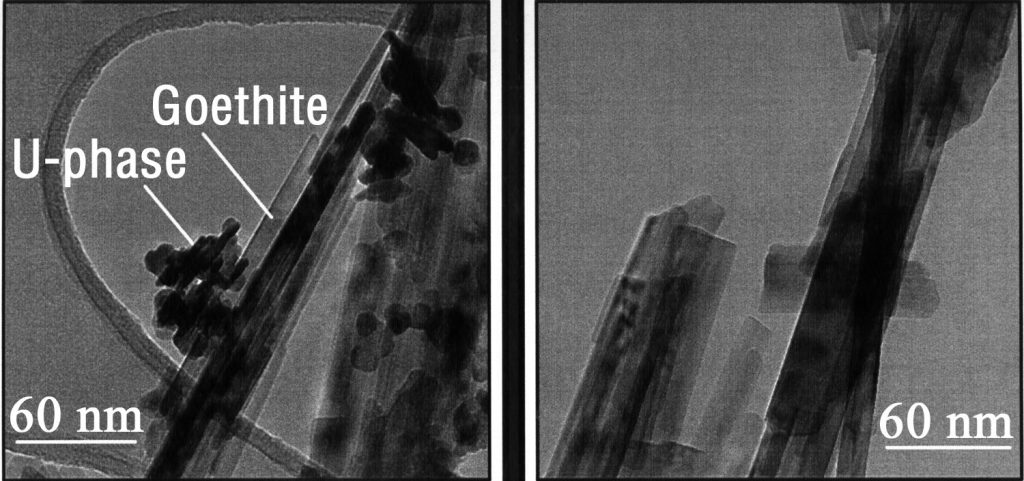
Download 300dpi JPEG image, ‘2uranium.jpg’, 224K (Media are welcome to download/publish this image with related news stories.)
Pat Brady, a Sandia geochemist who led the LDRD project and has studied many uranium-contaminated sites in the world, says natural attenuation is good for some but not all uranium and heavy metals clean-up efforts.
“It all depends on risk to humans and environmental health,” he says. “Some sites are so dangerous you can’t wait for natural attenuation to decrease the levels of toxins. But in other cases it may be best to let nature take its course. Clean-up efforts, because they are expensive and because they often provide their own health risks, shouldn’t be done if a real health benefit cannot be demonstrated.”
Brady gives an example of the radioactive isotope 137Cs (a cesium atom with 137 protons and neutrons in its nucleus) which was released, most notably from Chernobyl, but which is rapidly removed from groundwater by mineral surfaces. For this reason the health risk associated with it is often decreased.
Sites contaminated with uranium pose special problems for the natural attenuation approach. Whereas natural attenuation of organic contaminants means breakdown and elimination by microorganisms, natural attenuation of metals involves encasing the radionuclides in a mineral where they won’t escape unless chemical conditions change dramatically.
Natural attenuation of heavy metals and radionuclides is referred to as “irreversible adsorption.” Contaminants can become irreversibly adsorbed through a number of processes, including incorporation of a heavy metal into a mineral in the soil or an aquifer or by being entrapped in a rock pore.
For example, uranium in groundwater is attracted to natural iron coatings located on walls of rocks through which the water is flowing. The radionuclides bind to the iron coating and then move into microscopic rock pore where they become sealed in and get incorporated into the iron coating. The radionuclides can no longer be “plucked up” by groundwater.
In studying how and if this is an effective method of cleaning up uranium-contaminated sites, the Sandia researchers conducted a number of complementary studies. These include laboratory work, computer simulations, and analysis of historical water quality data from uranium-contaminated sites.
Questions asked
The scientists also have sought answers to several key questions: the rate and extent of irreversible adsorption of uranium, what fraction of uranium in aquifers is associated with the phenomenon, and how much irreversible adsorption of uranium is required to reduce the amount of uranium that eventually could reach humans to “safe” levels.
While scientists are aware that irreversible adsorption occurs, they face one potential problem. Contaminated sites must be monitored to determine how rapidly the uranium is being removed through this method. Long-term monitoring is expensive and it becomes a question of whether the cost exceeds that of traditional clean-up methods. Cheaper and better designed systems and methods of monitoring must be developed and are being investigated by several researchers throughout Sandia.
“This is a long-term work in process,” Siegel says. “We are now doing an analysis to see if this irreversible adsorption happens fast enough. The natural method is preferable, and we are trying to establish an approach that can be used at all sites.”
