ALBUQUERQUE, N.M.— Basic assumptions about water’s adsorption to platinum do not hold true, Sandia researchers have found.
“The way that water molecules prefer to arrange themselves on platinum has always been largely a matter of speculation,” Sandia researcher Peter Feibelman said.
Accurate knowledge is important because the first layer of water molecules at the surface of a solid determines how easily water can flow past, guides the growth of ice crystals, and plays an important role in surface chemical phenomena. The metal is, among other things, a widely used catalyst.
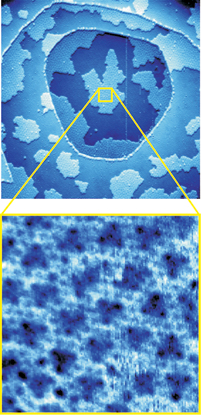
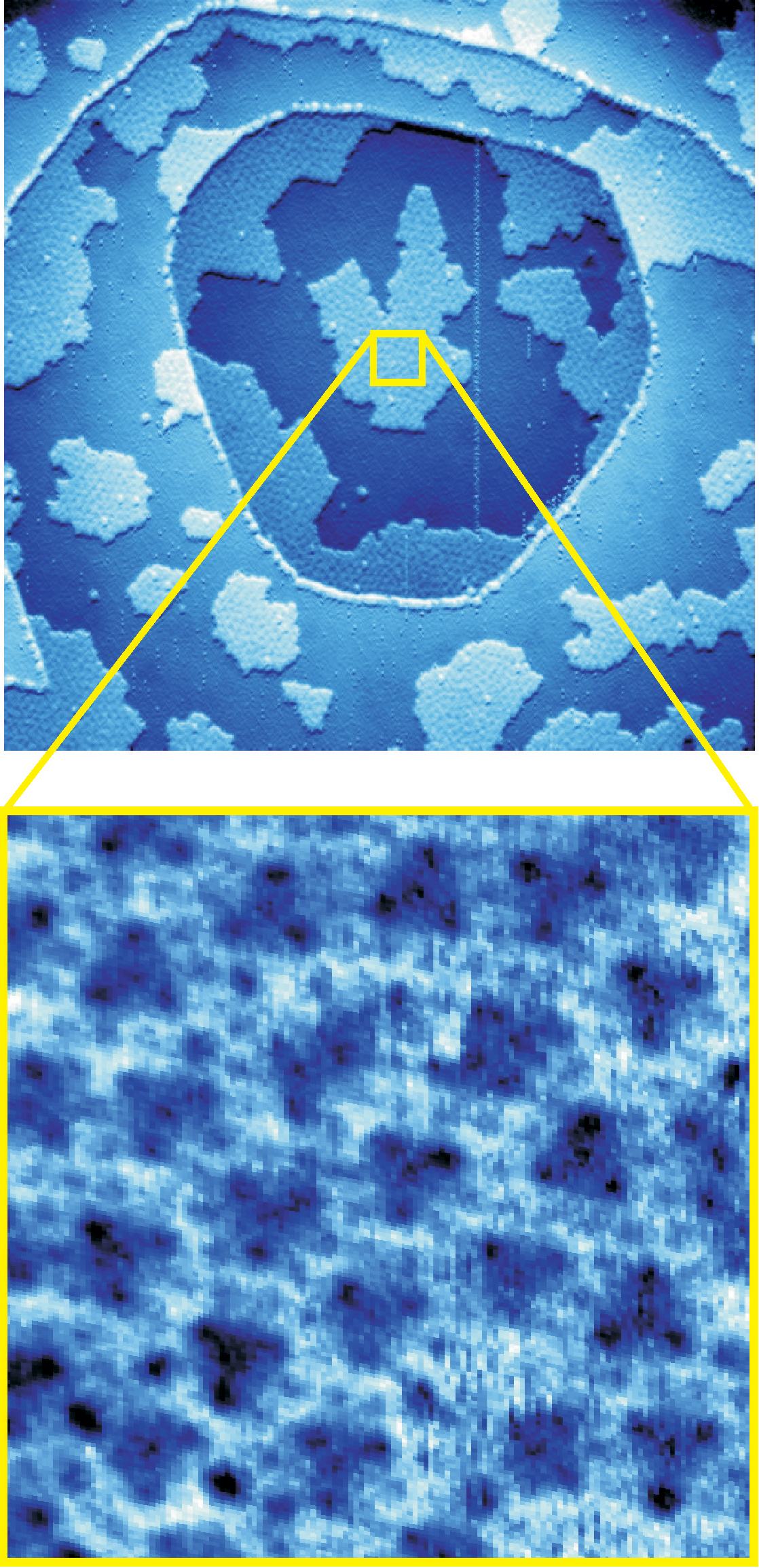
The Sandia group present a radically new picture by working at ice-forming temperatures (to stabilize the interaction long enough to view molecular details with a scanning tunneling microscope), and then basing computer simulations on the results.
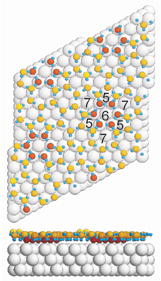
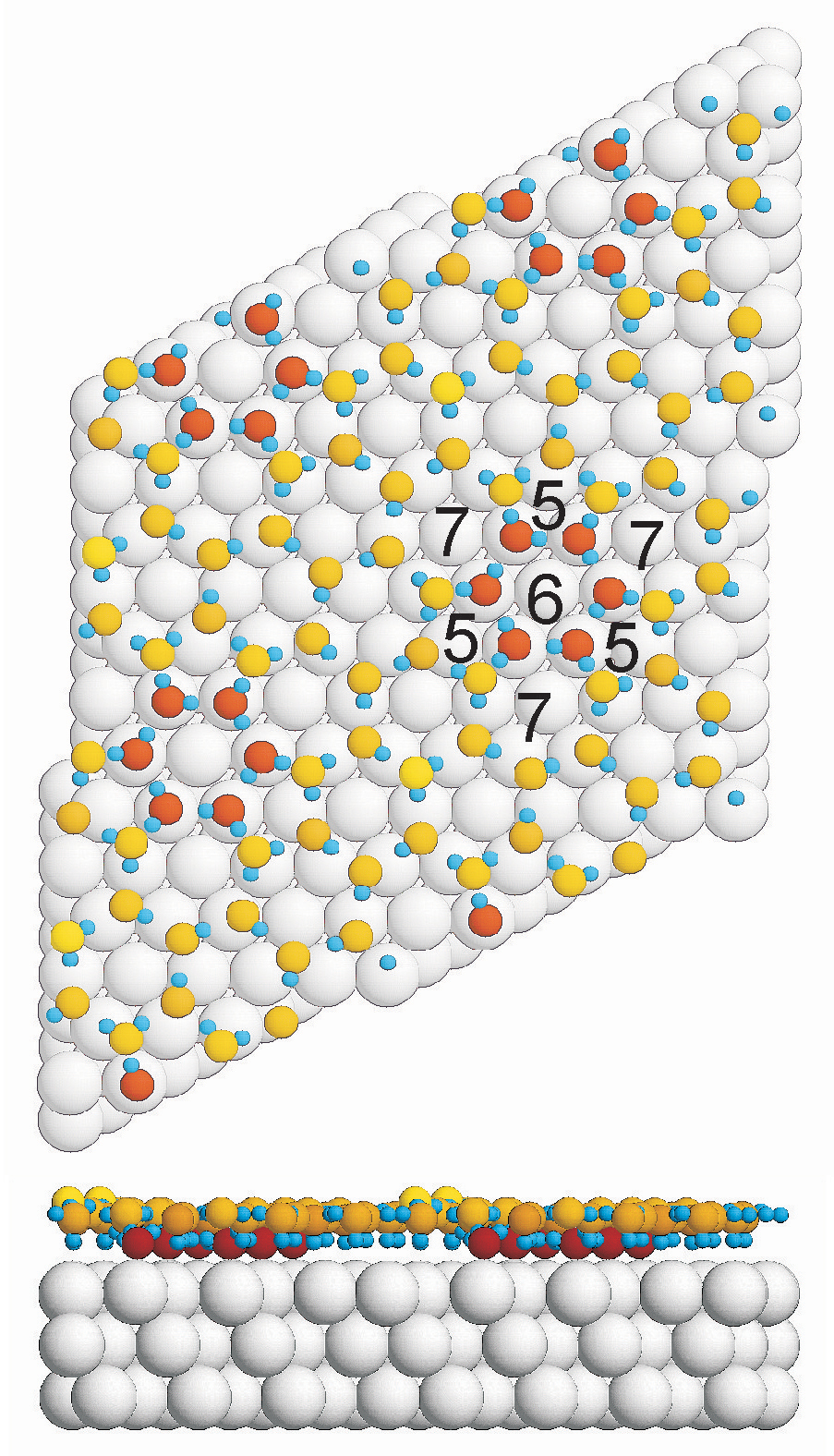
They found that to exist in the state of least energy – the endpoint sought by all undisturbed materials — water molecules arrange themselves in pentagons and heptagons in the primary wetting layer, not hexagons as their well-known arrangement in ice crystals suggests.
The unexpected result “underlines the importance of directly characterizing the first stages of water adsorption before claiming that one understands how water interacts with solids,” the researchers write in an article published July 9 in Physical Review Letters.
The Sandia team of Shu Nie, Norm Bartelt, Konrad Thürmer and Feibelman began with 1997 experimental results from the University of Göttingen in Germany. Those experiments showed that water initially grows as two-dimensional crystals on platinum, unexpectedly rotated so that they seem poorly aligned with the metal atoms.
Formation of a two-dimensional wetting arrangement has the well-understood meaning that water molecules are more attracted to platinum atoms than to each other. But why the rotation?
High-resolution scanning-tunneling microscope images of the delicate system led the Sandia researchers to attribute the rotation to a clustering of water molecules lying parallel to the metal surface in a way that allows the molecules at the center of the cluster to bind particularly strongly to the metal, becoming, in effect, “molecular anchors.”
The theoretical model proposed by the researchers added the surprising detail that the anchors’ connections to the rest of the wetting layer is through water molecules arrayed in pentagons and heptagons, not hexagons.
“We think the pentagon/heptagon arrangement allows bonds to bend down, connecting higher-lying with lower-lying molecules in a relatively strain-free way,” Feibelman said. “This also suggests, however, that 3-D islands will not grow atop the wetting layer without substantial molecular rearrangement.”
A similar scenario is likely true for water on other metal crystal surfaces, he said. “More image data and more calculations will clarify the picture, probably pretty soon.”
Whether there are lessons from the metal studies that carry over to oxides and other insulators is an interesting question, “one I am drawn to,” Feibelman said. “We hope our results will yield a picture of value, down the track, for applications, but are happy enough to have made the progress we did.”
He notes that water on platinum has become a model system over time because scientists have learned to prepare highly perfect, extremely pure platinum surfaces, and developed tools to study molecules deposited on them.
The work was supported by the Department of Energy’s Office of Basic Energy Sciences.