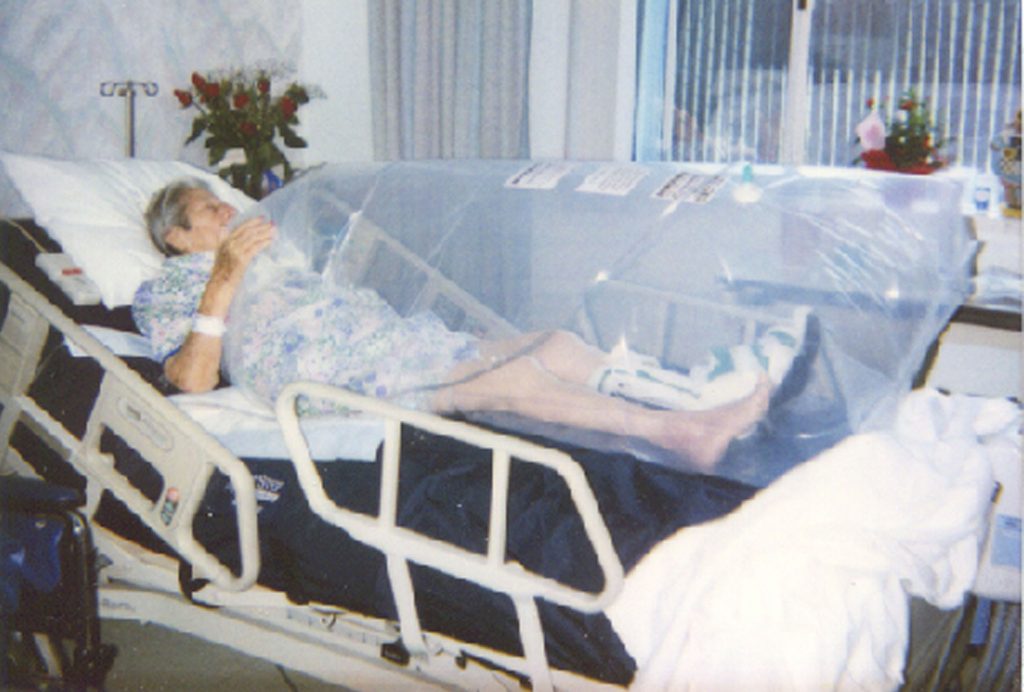
ALBUQUERQUE, N.M. — A disposable plastic bag resembling the common kitchen garbage bag, its interior fed by a simple oxygen canister monitored by inexpensive, deceptively simple plastic instruments, has been licensed by the federal government as a tool to heal the sick and the wounded in the nation’s military, both active and retired.

The product — called the Numobag™ after its creator and distributor, Numotech Inc. of Northridge, Calif. — was made commercially viable with the aid of inexpensive sensors and safety features developed at Sandia National Laboratories.
The heightened oxygen content helps oxidize, or burn up, organisms on the skin or in wounds, in addition to helping flesh itself heal. Tests of the bag have produced clinical evidence that the technique acts to minimize scarring and shorten treatment times for skin wounds, such as pressure ulcers, diabetic foot ulcers, severe burns, and plastic surgery.
The mobile, low-cost technique is of further interest to the military because it is also considered an effective treatment for smallpox and dermal anthrax.
“In other words,” says Ray Shaum, a senior administrator at Sandia, “the same characteristics that make Topical Hyperbaric Oxygen Therapy (THOT) an effective treatment regimen for diabetes-related necrotizing fasciitis make it effective for treating biological-warfare related lesions.”
In extreme cases, the tool could be used as a personal, inexpensive, and disposable isolation ward for the person being treated.
Because the process kills bacteria, expensive hospital disposal procedures are not needed and the bags are discarded as simple trash.
The official acceptance opens a huge guaranteed market for the product. The Numobag is currently used in hospitals in Florida and California, soon will be used at University of New Mexico Hospital, and by early 2004 is expected to be on the market in simplified form so that it can be used by patients in the comfort of their own homes. The home model is expected to eliminate the need for freestanding oxygen tanks by extracting oxygen directly from the atmosphere.
The Sandia research team was led by Mark Vaughn and Keith Miller. Sandia and Numotech each hold patents on the sensors and the Numobag, respectively, the result of their work under Sandia�s cooperative research and development agreement.
According to Dr. Robert Felton, founder, CEO, and president of Numotech, “We relied on Sandia Labs highly for its technical contributions. Without those, we could never have commercially produced the Numobag. We’re now capable of responding to the government for their needs for armed conflicts with a production of 50,000 Numobag Kits a month.”
The relatively new medical tool offers an inexpensive alternative to the solid, room-like constructions found in some hospitals. These similarly administer oxygen at higher concentration levels than ordinary air normally provides to stimulate wound healing. Such facilities are expensive to build, with capital costs of approximately $1 million, and are costly to maintain. They require extensive cleaning after each use, and require total immersion of the patient in the oxygen-enriched chamber. Costs for an oxygen treatment in such facilities can reach $1,500, while the Numobag’s estimated cost per treatment is $185, according to figures provided by MR Beal Inc., a New York-based investment house providing backing for the venture.
THOT is applied directly (and only) over the injured part, leaving the patient free to interact with his or her ordinary environment.
Says Vaughn, “The big thing is its ease of use. We developed helpful technology for making the Numobag more useful with our inexpensive, miniaturized pressure sensors. Now, with the official designation that this is a viable therapy, the Veterans Administration, Medicare-Medicaid, the Navy, the Army, the Air Force can say, ‘we want to put these on our boats or whatever, and we can buy a bunch of them.’”
Says Sandia tech transfer expert Gordon Leifeste, “What Numotech achieved with its persistence is very important. Programs like Medicare are heavily regulatory-driven. Now the device has a worldwide federal contract. That’s effectively a DLA [Defense Logistics Agency] number, which is hard to come by. It’s important because you might think you’ve invented a better mousetrap but you might not be able to sell it because you’re constrained by the federal regulatory environment: the federal agencies haven’t given you a number. Now this device is one of the relatively few that physicians can choose from that have won government approval.”
Says Ray, “The DLA places blanket contracts that anyone in the government (e.g. Sandia, FEMA, VA, DoD) can utilize. They are similar to contracts in which pricing is already established, and buyers do not need to justify the selection. The benefit to Numotech is dual: 1) anyone in a government agency can now buy the Numobag Kit without having to justify the purchase as a competitive acquisition; and 2) DLA exhaustively checked out the medical efficacy claims of THOT. Award of a contract validates claims that might otherwise be seen as marketing hype.”
The Numobag kits, produced in California, are of extruded polyethylene.
“Each is cleaned to specifications equivalent to a class 1000 clean room,” says Felton, “and the product does not outgas when used” The problem of out gassing, a potentially serious drawback, was overcome by researchers, as was the problem of bonding the throwaway pressure gauge to polyethylene, a substance that sheds adhesives.
“The sensors are simple but not easy,” said Vaughn.
The external pressure gauge developed by Vaughn and Miller tests the tension of the Numobag wall, rather than puncturing it to directly test gas pressure. Because the Sandia researchers knew that the tension of the polyethylene surface was proportional to the internal pressure of the gas, they could create a simple system that showed whether gas pressure was high, low, or just right. With this simple indicator, the pressure could by regulated by healthcare providers such as nurses.
Sandia expects to work further to produce a colorimeter that will assess the progress of wound healing, as well as a cheap device to measure the quality of wound out gassing. The device would be similar to those used to detect changes in stored nuclear weapons, says Vaughn.
The idea of the Numobag and its first tests were achieved by Madalene Heng, chief of dermatology at the VA center at Sepulveda, Calif., and professor at the University of California School of Medicine. Heng has conducted, published, and presented extensive research on wound care. While the method proved effective, it relied on her skilled presence to instruct nurses on the conditions necessary in each Numobag. Sensors created by Sandia are expected to do that job, permitting less skilled personnel in widely scattered geographic areas to use the healing device.
Dr. Glen Heywood, a professor of surgery at the Health Sciences Center at the University of New Mexico, says that he is “gearing up to try the Numobag for the most serious wound infections we deal with as surgeons — necrotizing fasciitis. There have been studies — some conflicting — but the studies seem to support that topical hyperbaric oxygen therapy may be helpful. We have in New Mexico one of the highest amounts of necrotizing fasciitis, maybe because our oxygen content is lower because of our higher altitude. We are the closest clinical institution in closest proximity to Sandia, where a significant portion of the engineering research has been done. Most of the clinicians are located in California. So it seemed appropriate for us to be a test site for the technique. Our aim is to achieve an objective randomized evaluation; it’s important that we clearly compare the efficacy of the Numobag with other techniques to find which wounds this technique operates on most effectively.”
The Numobag is the third project the company has undertaken with Sandia. The others are a wheelchair seat and wheelchair back. Each use inflatable air pockets with miniaturized controls and pumps to reduce the possibility of pressure sores for quadriplegic and diabetic patients. Pressure sores are open, chronic lesions that are difficult to heal and can lead to amputation or even death.
For further information on this project, see the earlier Sandia Lab News story at http://www.sandia.gov/LabNews/LN06-19-98/oxygen_story.html.
