ALBUQUERQUE, N.M. — Steel pipes rust and eventually fail. To preempt disasters, oil companies and others have created computer models to predict when replacement is needed. But if the models themselves go wrong, they can be modified only through experience, a costly problem if detection comes too late.

Now, researchers at Sandia National Laboratories, the Department of Energy’s Center for Integrated Nanotechnologies and the Aramco Research Center in Boston, have found that a particular form of nanoscale corrosion is responsible for unpredictably decreasing the working life of steel pipes, according to a paper recently published in Nature’s Materials Degradation journal.
Using transmission electron microscopes, which shoot electrons through targets to take pictures, the researchers were able to pin the root of the problem on a triple junction formed by a grain of cementite — a compound of carbon and iron — and two grains of ferrite, a type of iron. This junction forms frequently during most methods of fashioning steel pipe.
Iron atoms slip-sliding away
The researchers found that interfacial disorder in the atomic structure of those triple junctions made it easier for the corrosive solution to remove iron atoms along that interface.
In the experiment, the corrosive process stopped when the triple junction had been consumed by corrosion, but the crevice left behind allowed the corrosive solution to attack the interior of the steel.
“We thought of a possible solution for forming new pipe, based on changing the microstructure of the steel surface during forging, but it still needs to be tested and have a patent filed if it works,” said Sandia’s principle investigator Katherine Jungjohann, a paper author and lead microscopist. “But now we think we know where the major problem is.”
Aramco senior research scientist Steven Hayden added, “This was the world’s first real-time observation of nanoscale corrosion in a real-world material — carbon steel — which is the most prevalent type of steel used in infrastructure worldwide. Through it, we identified the types of interfaces and mechanisms that play a role in the initiation and progression of localized steel corrosion. The work is already being translated into models used to prevent corrosion-related catastrophes like infrastructure collapse and pipeline breaks.”
To mimic the chemical exposure of pipe in the field, where the expensive, delicate microscopes could not be moved, very thin pipe samples were exposed at Sandia to a variety of chemicals known to pass through oil pipelines.
Sandia researcher and paper author Khalid Hattar put a dry sample in a vacuum and used a transmission electron microscope to create maps of the steel grain types and their orientation, much as a pilot in a plane might use a camera to create area maps of farmland and roads, except that Hattar’s maps had approximately 6 nanometers resolution. (A nanometer is one-billionth of a meter.)
“By comparing these maps before and after the liquid corrosion experiments, a direct identification of the first phase that fell out of the samples could be identified, essentially identifying the weakest link in the internal microstructure,” Hattar said.
Sandia researcher and paper author Paul Kotula said, “The sample we analyzed was considered a low-carbon steel, but it has relatively high-carbon inclusions of cementite which are the sites of localized corrosion attacks.
“Our transmission electron microscopes were a key piece of this work, allowing us to image the sample, observe the corrosion process, and do microanalysis before and after the corrosion occurred to identify the part played by the ferrite and cementite grains and the corrosion product.”
When Hayden first started working in corrosion research, he said, “I was daunted at how complex and poorly understood corrosion is. This is largely because realistic experiments would involve observing complex materials like steel in liquid environments and with nanoscale resolution, and the technology to accomplish such a feat had only recently been developed and yet to be applied to corrosion. Now we are optimistic that further work at Sandia and the Center for Integrated Nanotechnologies will allow us to rethink manufacturing processes to minimize the expression of the susceptible nanostructures that render the steel vulnerable to accelerated decay mechanisms.”
Invisible path of localized corrosion
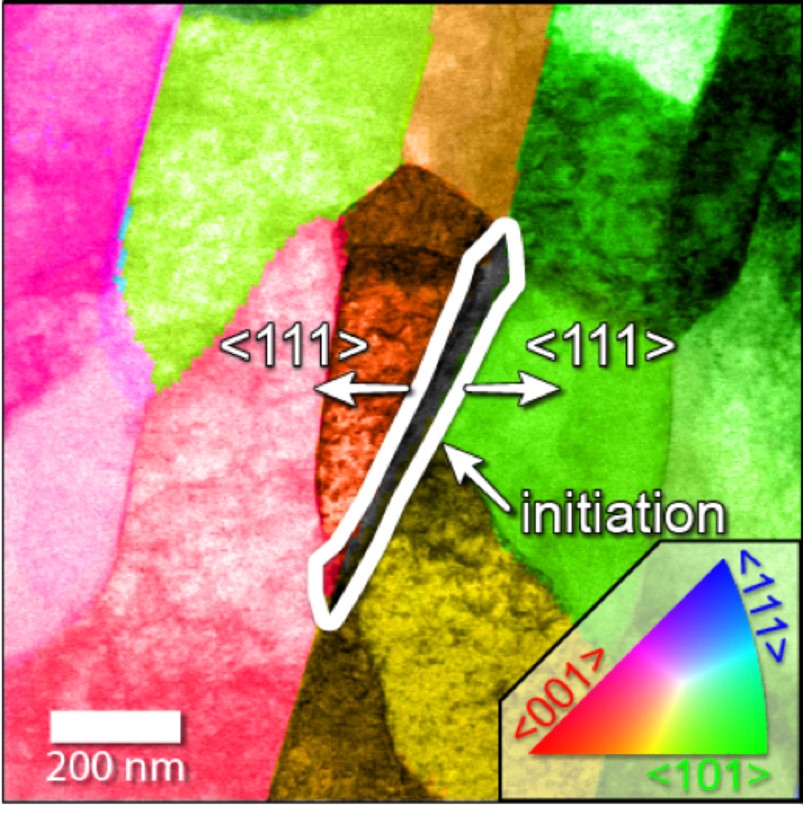
Localized corrosion is different from uniform corrosion. The latter occurs in bulk form and is highly predictable. The former is invisible, creating a pathway observable only at its endpoint and increasing bulk corrosion rates by making it easier for corrosion to spread.
“A better understanding of the mechanisms by which corrosion initiates and progresses at these types of interfaces in steel will be key to mitigating corrosion-related losses,” according to the paper.
Other authors include Sandia researchers William Mook and Daniel Bufford and former Sandia researcher and experimental lead Claire Chisholm, who is now at the University of California at Santa Barbara.
The work was funded in part by Sandia’s Laboratory Directed Research and Development program. The Center for Integrated Nanotechnologies is a DOE Office of Science user facility operated by Sandia and Los Alamos national laboratories for university, industry and other national lab researchers.
