LIVERMORE, Calif. — Sandia National Laboratories researchers have identified key chemical mechanisms for the first time that add to the fundamental knowledge of combustion chemistry and might lead to cleaner combustion in engines.
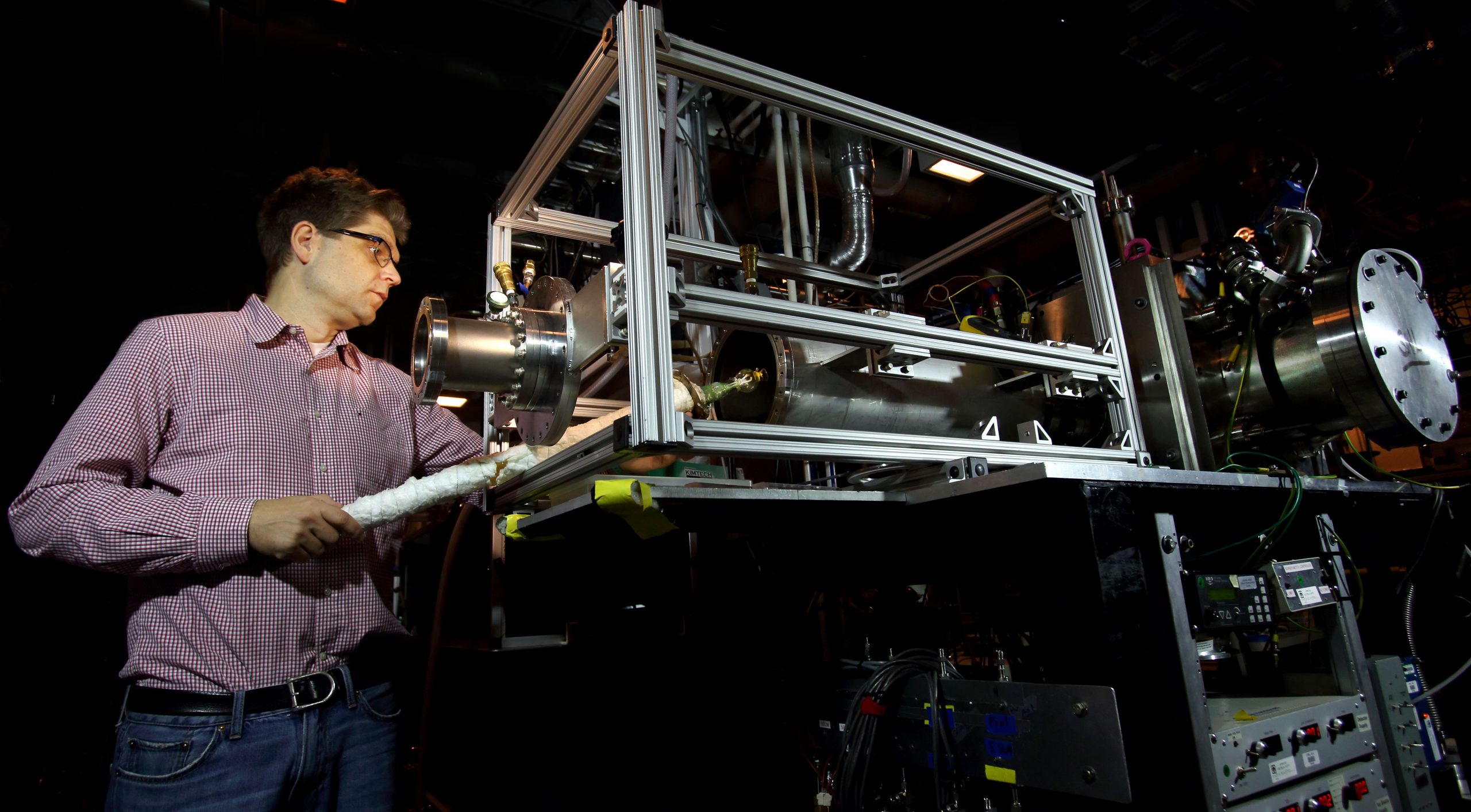
Sandia researcher Nils Hansen and former postdoctoral appointee Kai Moshammer focused on low-temperature oxidation of hydrocarbons and other alternative fuels. They identified key chemical intermediates, which are relevant for oxidation reactions at temperatures in the range of 400 to 600 K (260 to 620 degrees Fahrenheit). The chemical nature of the intermediates and their concentrations provides new details on the chemical processes involved in autoignition.
Autoignition is a chemical process in which a fuel-air mixture spontaneously ignites. It is commonly explained by theory through a set of self-sustaining and accelerating chain-branching reactions. It is most important for understanding knock in spark-ignition engines.
Hansen and Moshammer were among a multi-institution team of researchers whose work was published in a paper titled, “Unraveling the structure and chemical mechanisms of highly oxygenated intermediates in oxidation of organic compounds.” The researchers focused on deepening the insights into low-temperature oxidation chemistry of hydrocarbons and other alternative fuels.
“We can run an internal combustion engine today without knowing the details of the chemistry,” Hansen said. “However, this new knowledge provides new insights that should be targeted by new combustion models. It eventually should allow for the development of more clean and efficient combustion strategies in the future.”
Hansen and Moshammer used molecular-beam mass spectrometry to discover the chemical intermediates. The molecular beam freezes the chemistry and can be compared to the German autobahn.
“In the molecular beam, all the molecules are sucked into a vacuum to fly in the same direction and at the same speed, so there are no collisions just like on the autobahn,” he said. “When we isolate the molecules this way, it allows us to separate them by their weight and thus their molecular makeup.”
Extracting detailed information from nature
Extracting detailed molecular information directly from igniting mixtures is a difficult and challenging task especially because of large temperature swings and the low molecular concentrations of key intermediates.
“Even after a few decades of research on this topic, these highly oxygenated molecules had never been seen before,” Hansen explains.
Yiguang Ju, professor and director of Sustainable Energy at Princeton University, said this work clearly reveals the formation of oxygenated intermediates through the multiple oxygen molecules addition processes. “The oxygenated intermediates are critical to affect low-temperature ignition, cool flame, mild flame and knocking formation in internal combustion engines,” Ju said.
Jet-stirred reactor designed to conduct research
Hansen stressed that these discoveries were made by experiments that focus on chemistry while minimizing the effects of mixing, turbulence and large temperature and concentration gradients.
To conduct the work, the Sandia researchers designed a device called a jet-stirred reactor, which is best described as a quartz reactor into which unburned fuel-oxidizer mixtures are continuously added through four small nozzles to create a homogenous mixture that is then ignited with external heat. With this approach, the researchers avoid large spatial and temporal changes in the concentrations of the key intermediates and temperatures and the reactor can be readily modeled. The researchers then used molecular-beam sampling and high-resolution mass spectrometry to identify gas components from the reactor.
“Our persistent interest in low-temperature oxidation processes led to this research,” Hansen said. “While the first studies focused around small fuels such as dimethyl ether (DME, CH3OCH3), we eventually moved to larger, more practically relevant fuels, such as heptane, and ‘accidentally’ detected a signal that was not explainable through the known chemical mechanisms. We wanted to provide validation targets for model developments in the form of molecular identification and concentration.”
Previous research identified reactions and intermediates that helped predict ignition characteristics of individual fuels. Sandia’s work has shown that the scientific community’s understanding of these processes is not complete and that additional reactions and intermediates must be considered. This work will help to develop models with better predictive capabilities, and has implications beyond combustion.
“This is fundamental chemical kinetics research that can also impact climate-relevant tropospheric aerosol formation,” Hansen said.
Paul Wennberg, the R. Stanton Avery professor of Atmospheric Chemistry and Environmental Science and Engineering at Caltech, said this research also provides a wealth of new data and insight into the oxidation processes involved in the oxidation of organic molecules in the atmosphere. For example, the knowledge of how many oxygens are added following the formation of the first radical, how the structures of the organic substrates alter the pathways, and whether this chemistry can compete with bimolecular processes is essential for predicting if this chemistry is important at the much colder temperatures relevant for the atmosphere.
“The final impact of these discoveries in autoxidation on our understanding of air pollution is unclear,” Wennberg said. “We know that breathing particulate is a public health threat, but how toxic the particulates created via autoxidation are and how long these compounds persist in the atmosphere is simply not known at this time.”
The use of mass spectrometry to detect these intermediates is only the first step in this research.
“In the future, we will need to develop new experimental techniques and capabilities that would allow for an unambiguous assignment of the molecular structure,” Hansen said. “We will test two-dimensional mass-spectrometric techniques and microwave spectroscopy as analytical tools to find the exact chemical structures.”
